Diagnosis of acute ischemic stroke
Clinical Manifestations and Lesion Localization
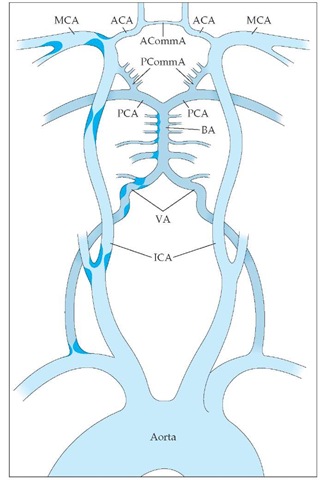
Two pairs of vessels supply blood to the brain: the internal carotid arteries and the vertebral arteries. These vessels, which deliver 20% of the cardiac output, join on the ventral surface of the brain to form the intracranial vessels and the circle of Willis [see Figure 5]. The anterior cerebral artery supplies blood to the medial frontal and deep structures. Occlusion of the anterior cerebral artery is characterized by contralateral leg weakness [see Table 2], but isolated infarction of the anterior cerebral artery is uncommon.
The middle cerebral artery (MCA) divides into two major trunks, and each trunk divides into five to seven branches that supply blood to the lateral hemisphere. Because the MCA supplies a large territory, MCA occlusion causes a clinical syndrome that includes contralateral hemiparesis and hemisensory deficit (in which the deficit in the face and arm is greater than the deficit in the leg), aphasia (if the dominant hemisphere is affected) or neglect (if the nondominant hemisphere is affected), contralateral visual-field defect, deviation of gaze, dysarthria, and other cortical symptoms.
Table 1 Differential Diagnosis of Acute Ischemic Stroke
|
Possible Cause |
Comment |
|
Drugs or other toxins |
Unlikely to cause focal neurologic symptoms; exclude by history and toxic screening |
|
Seizure |
Can mimic focal neurologic signs; exclude by history |
|
Metabolic derangements |
Abnormalities of glucose, calcium, PO2, PCO2, and electrolytes and liver and kidney dysfunction can all cause neurologic abnormalities; hypoglycemia and hyperglycemia are notorious for causing focal signs; exclude by laboratory tests |
|
Migraine |
Although migraine is a diagnosis of exclusion, it must be considered in patients with stroke; headache can be a prominent component of both ischemic and hemorrhagic stroke; exclude by history and physical examination |
|
Brain tumor |
Unlikely to present acutely; exclude by history and CT scan |
|
Intracranial hemorrhage |
Subdural, epidural, subarachnoid, and intracere-bral hemorrhage can all mimic ischemic stroke; exclude by CT scan |
|
Psychiatric disease |
Conversion disorder and malingering can usually be discovered by a careful physical examination and history |
CT—computed tomography
PCO2—carbon dioxide tension
PO2—oxygen tension
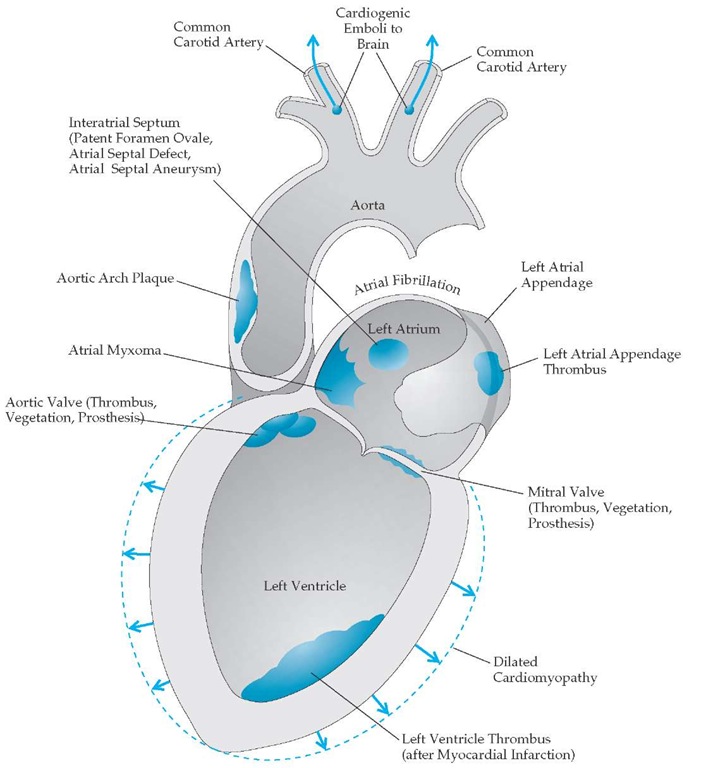
Figure 4 Potential sources of cardioembolism.
The two major branches of the vertebral arteries are the anterior spinal artery, which supplies the spinal cord, and the posterior inferior cerebellar artery, which leads to the inferior cerebellum and the lateral medulla. The two vertebral arteries then unite to form the basilar artery. The major branches of the basilar artery are the anterior inferior cerebellar artery and the superior cere-bellar artery, which supply parts of the pons and cerebellum. Occlusion of the vertebral arteries or basilar artery leads to a combination of signs and symptoms that depend on the level and extent of infarction. These signs and symptoms include so-called crossed facial sensory and body motor signs, diplopia, facial numbness and weakness, vertigo, nausea and vomiting, tinnitus, hearing loss, ataxia, gait abnormality, hemiparesis, dysphagia, and dysarthria. The basilar artery terminates by dividing into two posterior cerebral arteries that supply the medial temporal lobe, the occipital lobe, and parts of the thalamus. Occlusion of the posterior cerebral artery results in occipital infarction and therefore contralateral visual-field loss. Such occlusion may also cause contralateral hemiparesis and behavioral changes.
After leaving the circle of Willis, the vessels branch repeatedly and ultimately become end arteries. Occlusion of these penetrating vessels typically manifests as pure motor hemiparesis, pure sensory stroke, clumsy hand-dysarthria syndrome, or ataxic hemiparesis.
Diagnostic Evaluation
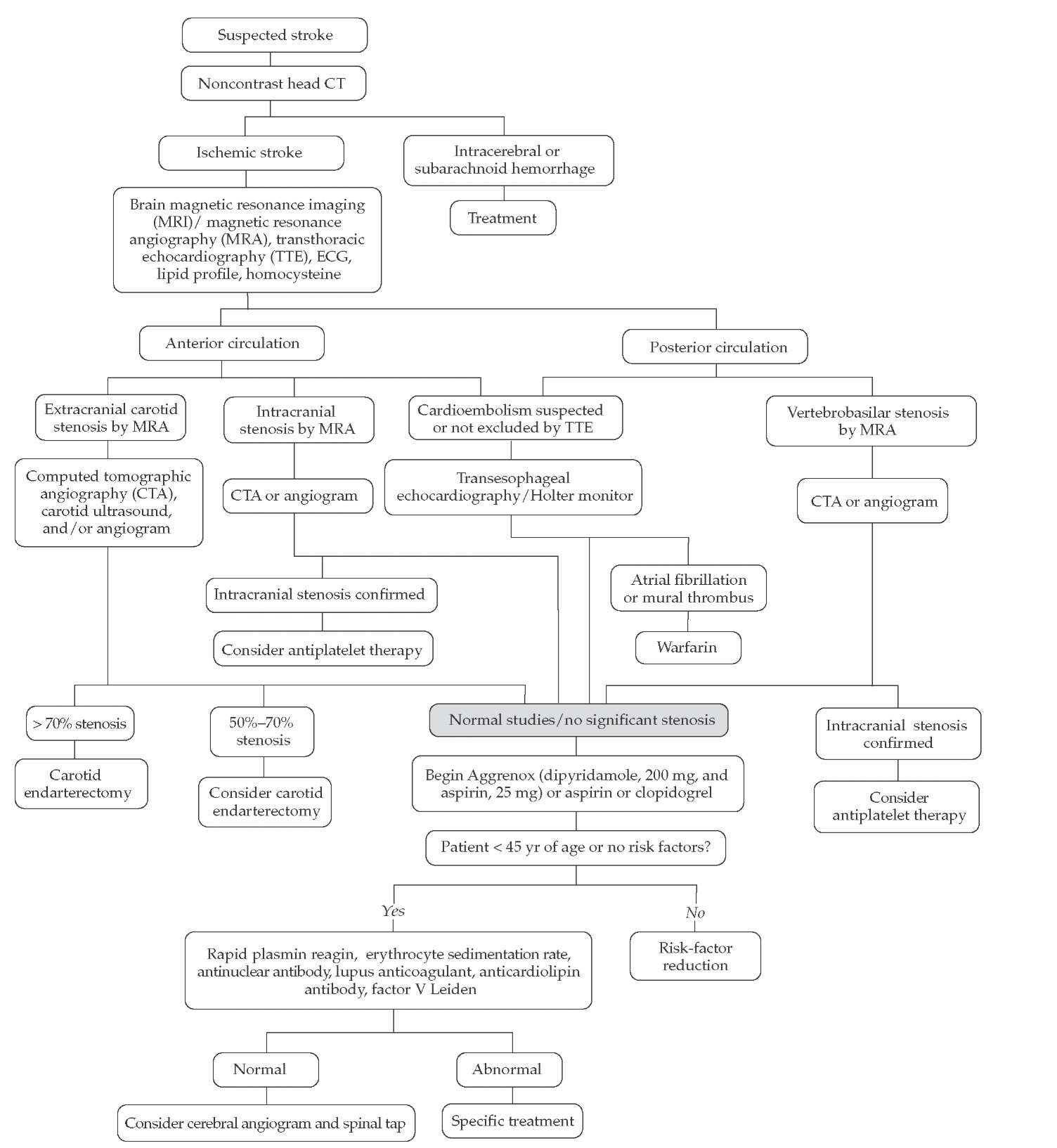
The highest risk of recurrent stroke occurs within the first month after initial stroke symptoms (and within the first few days after TIA)13,14; therefore, an expeditious evaluation of patients presenting with TIA or suspected stroke should be undertaken and prophylactic therapy begun immediately at presentation [see Figure 6] (though after initial treatment with thrombolytics, if applicable [see Treatment of Acute Ischemic Stroke, below]). In a patient with recent cerebral ischemia, the first step is to localize the lesion [see Clinical Manifestations and Lesion Localization, above]. Patients with anterior circulation strokes should undergo evaluation of the heart, extracranial carotid arteries, and intracranial anterior circulation. Cardiac evaluation [see Figure 4] begins with an electrocardiogram, a cardiac history and examination, and either a transthoracic echocardiogram (TTE) or a transesophageal echocardiogram (TEE). If a TTE does not provide an absolute diagnosis but suggests mural throm bus, valvular disease, or patent foramen ovale, then a TEE is ordered. A TEE allows visualization of structures not seen on a TTE, including clots in the left atrial appendage and aortic arch. The extracranial carotid circulation and intracranial anterior circulation can be visualized by MRA or CTA. In hospitals where these diagnostic tools are either not available or of poor quality, carotid ultrasonography should be performed. Transcranial Doppler imaging can help detect intracranial stenosis. The gold standard remains conventional cerebral angiography. Because this invasive test has possible complications (1% risk of stroke in most series) and is expensive, it should be reserved for cases in which its results could change treatment decisions. Posterior circulation evaluation involves the same cardiac evaluation. The posterior circulation is visualized by MRA, CTA, or conventional angiography.
Figure 5 Cerebrovascular anatomy and common sites of atherosclerosis are shown. The internal carotid artery (ICA) enters the skull, and its first major branch is the ophthalmic artery to the eye. Next are the anterior choroidal artery and the posterior communicating artery (PCommA). The PCommA connects the anterior circulation to the posterior circulation. The ICA then terminates as it divides into the anterior cerebral artery (ACA) and the middle cerebral artery (MCA). The vertebral arteries (VA) enter the skull and merge at the inferior border of the pons to form the basilar artery (BA). The BA then terminates as it divides into the two posterior cerebral arteries (PCA). (ACommA—anterior communicating artery)
Subcortical infarctions, or lacunae, that are greater than 1.5 cm in diameter are usually thromboembolic in nature. The evaluation in these patients is the same as that described above.
Laboratory investigations for all patients include measurement of the fasting lipid level within 48 hours of symptom onset, measurement of the homocysteine level, complete blood count, prothrombin time, partial thromboplastin time, and chemistry panel. If the patient is younger than 45 years or has no stroke risk factor or other identified etiology, the following tests should be considered but are of low yield: anticardiolipin antibody test, lupus anticoagulant profile, erythrocyte sedimentation rate, testing for factor V Leiden, rapid plasmin reagin test, and antinu-clear antibody test. Tests for rarer causes of ischemic stroke include assays for protein C and protein S, measurement of an-tithrombin III, testing for prothrombin gene mutation, testing for HIV, and measurement of lactate level (for mitochondrial disease). Additionally, lumbar puncture and cerebral angiogra-phy should be considered if there is suspicion of infection or inflammation of the cerebral blood vessels.
Treatment of acute ischemic stroke
Antiplatelet and Antithrombotic Treatment
Aspirin (160 to 325 mg daily) administered within 48 hours of stroke onset has been shown to significantly reduce the risk of recurrent stroke during the first 2 weeks and, possibly, to improve outcome at 6 months.15,16 Therefore, aspirin is recommended as initial therapy for most acute stroke patients. However, aspirin should be withheld for at least 24 hours after administration of thrombolytics [see Intravenous Recombinant Tissue Plasminogen Activator, below]. For patients who have a known contraindication to aspirin, other antiplatelet agents may serve as rational, although unproven, alternatives.
Anticoagulation is commonly used in the acute setting to prevent progressive or recurrent thromboembolic events. Nevertheless, the efficacy and safety of anticoagulation for this purpose are not well established, and its role in clinical stroke management is controversial. Many neurologists formerly used heparin, although studies have demonstrated that it offers no appreciable benefit for most patients. The International Stroke Trial was a multicenter clinical trial involving 19,436 patients who were ran- domized within 48 hours of stroke onset into two arms. In the first arm, patients received subcutaneous heparin at a dosage of either 12,500 units or 5,000 units twice daily for 14 days. In the second arm, patients received no heparin.15 Patients were independently randomized to receive either 300 mg of aspirin daily or no aspirin. The rate of recurrent stroke or death at 14 days was 11.7% in the patients receiving heparin and 12.0% in the nonhep-arin group—an insignificant difference—and there was no improvement in outcome at 6 months. The reduction in ischemic strokes by heparin was completely counterbalanced by an increase in hemorrhagic strokes. Similarly, in randomized clinical trials, use of low-molecular-weight heparin (LMWH) did not lead to a reduction in recurrent stroke or improvement in out-comes.17 Therefore, as noted in a scientific statement developed jointly by the American Heart Association and the American Academy of Neurology, most patients with ischemic stroke should not be treated with anticoagulation.17 Some neurologists believe that carefully selected patients, such as those with acute basilar thrombosis, may benefit from acute anticoagulation therapy, although there is no clear evidence for or against its use.
Table 2 Clinical Features of the Major Cerebrovascular Occlusive Syndromes
|
Artery |
Major Clinical Features |
|
Anterior cerebral artery |
Contralateral leg weakness |
|
Middle cerebral artery |
Contralateral face + arm > leg weakness, sensory loss, visual-field cut, aphasia/neglect |
|
Posterior cerebral artery |
Contralateral visual-field cut |
|
Basilar artery |
Oculomotor deficits and/or ataxia with "crossed"sensory/motor deficits |
|
Vertebral artery |
Lower cranial nerve deficits and/or ataxia with "crossed" sensory deficits |
|
Penetrators |
Contralateral motor or sensory deficit without cortical signs* |
*Cortical signs include aphasia, apraxia, neglect, and other cognitive abnormalities.
Figure 6 Algorithm for the diagnostic evaluation of suspected stroke.
Intravenous Recombinant Tissue Plasminogen Activator
Intravenous recombinant tissue plasminogen activator (rt-PA) was approved for use in acute stroke by the Food and Drug Administration in 1996, and consensus statements from the American Academy of Neurology and the American Heart Association support its use.18 Although the FDA has approved the use of intravenous rt-PA for acute ischemic stroke up to 3 hours after the onset of symptoms, physicians should strive to treat patients as quickly as possible because earlier therapy is associated with better outcomes.19 In acute stroke patients who meet the criteria for its use [see Table 3], rt-PA is given in a dose of 0.9 mg/kg (maximum dose, 90 mg) infused over 1 hour, with 10% of the total dose infused over the first minute. If treatment with rt-PA is suspected of inducing intracranial hemorrhage, the infusion should be suspended.
Two major trials conducted by the National Institute of Neurological Disorders and Stroke led to FDA approval of rt-PA.19,20 The final outcome measure was return to independent function (i.e., no disability) at 3 months after onset of stroke. These studies together enrolled 624 patients at eight centers across the United States. Intravenous rt-PA was compared with placebo for patients presenting within 3 hours after onset of symptoms. The rate of symptomatic intracranial hemorrhage at 36 hours after administration of intravenous rt-PA was 6.5%, compared with 0.6% in the control group. Mortality from intracranial hemorrhage was 2.9% in the rt-PA group, compared with 0.3% in the placebo group.
Including the risk of intracranial hemorrhage, disability was significantly reduced when measured at 3 months, 6 months, and 1 year.21 The rt-PA-treated patients were at least 30% more likely to recover to independent function than the placebo-treated patients. Mortality at 1 year was 24% in the rt-PA group and 28% in the placebo group. This benefit was seen in all stroke subtypes regardless of age or patient risk factors. Patients with large strokes were more likely to experience ICH after use of intravenous rt-PA, but this group was also more likely to have severe disability or to die if left untreated.
Only a small proportion of stroke patients are currently treated with rt-PA. Aggressive public and professional education effectively increases this proportion.