Definitions
Stroke is a sudden neurologic deficit caused by either ischemia (80%) or hemorrhage (20%). Ischemic stroke is classified according to the area of the brain affected and the etiologic mechanism. Hemorrhagic stroke is classified as either subarachnoid (5%) or intracerebral (intraparenchymal) (15%). Transient is-chemic attack (TIA) is a sudden vascular-related focal neurologic deficit that resolves completely. TIAs are classically defined as lasting less than 24 hours; they generally last less than 1 hour. A TIA should not be considered a separate entity but, rather, a herald of ischemic stroke and an opportunity to intervene.
Epidemiology
Stroke is the leading cause of disability and the third leading cause of death in the United States. Until 1998, the annual incidence of stroke was estimated to be 550,000 on the basis of studies of homogeneous, predominantly white populations. Results of the Greater Cincinnati/Northern Kentucky Stroke Study suggest that previous reports underestimated stroke incidence by almost 50%. By rigorously counting strokes in all racial and ethnic groups in Cincinnati, the yearly estimate for the United States was revised to 731,100.* The explanation for the previous underestimation of stroke incidence is that the incidence is higher in African Americans than in non-Hispanic whites. African Americans in Cincinnati had a twofold to fourfold higher incidence of stroke than did non-Hispanic whites in Rochester, Minnesota, during the same period. In northern Manhattan, African Americans had a 2.4-fold higher incidence of stroke than did non-Hispanic whites.
African Americans also have higher stroke mortality. In Texas, African-American men 45 to 59 years of age had a 306% higher stroke mortality than did non-Hispanic whites. At 75 years of age and older, when stroke mortality is at its highest, the excess mortality for African-American men had diminished to 26% above the mortality for non-Hispanic whites. African-American women in the same age groups had a 222% and a 10% greater stroke mortality, respectively, than that of non-Hispanic whites.3 The increased burden of stroke in African Americans may be worsening. Between 1992 and 1996, rates of almost all subtypes of stroke had increased among African Americans, and the number of stroke deaths had increased by 8%.
The pathogenesis of stroke in African Americans may also differ somewhat from that in non-Hispanic whites. Extracranial carotid disease and cardioembolism more commonly cause is-chemic stroke in non-Hispanic whites, whereas intracranial thromboembolic disease is more common in African Ameri-cans.5 However, the various disease mechanisms can occur in either racial group.
Information on stroke in Hispanic Americans is less readily available. Data from northern Manhattan suggest a twofold higher incidence of stroke in Hispanics than in non-Hispanic whites.2 In Mexican Americans, the incidence of ischemic stroke and intracerebral hemorrhage is substantially greater than in non-Hispanic whites.6 As the Mexican-American population grows and ages, targeting of this population for stroke prevention becomes critical.
Women have lower stroke rates than men at all age ranges except 75 years and older, when stroke rates are at their highest. In Texas, 61% of all stroke-related deaths occur in women.3 Stroke and vascular disease have traditionally been seen as male disorders; this has resulted in a shift in the focus of prevention and acute treatment regimens away from women.
Overall, the decline in stroke-related mortality has inexplicably slowed over the past several decades and almost came to a halt in the 1990s.4 A lack of emphasis on prevention may have led to an increase in incidence, particularly in some racial and ethnic groups. As people survive other diseases, stroke may become a more common cause of death. Nonetheless, this leveling of the previous downward trend in stroke mortality is cause for alarm.
Approach to the Acute Stroke Patient
Stroke is the quintessential medical emergency. Stroke patients who receive neurologic care within 6 hours of the onset of symptoms have a fourfold greater chance of a good outcome than those treated after this acute period.7 This observation, which was made before the thrombolytic era, illustrates the crucial link between time and outcome for stroke victims. Patients who receive early treatment to restore cerebral perfusion and to maximize protection of neurons have better outcomes. Physicians can reduce the delay in getting stroke victims to the emergency department by educating at-risk patients and their families about stroke symptoms and encouraging them to call 911 if stroke symptoms occur.8 Once a stroke patient arrives at the emergency department, triage and treatment are critical in reducing mortality and morbidity [see Figure 1]. One clinical instrument for assessing stroke patients is the National Institutes of Health Stroke Scale, which is available online at http:/ / www.ninds.nih.gov/doctors/NIH_Stroke_Scale.pd. Before specific treatment is implemented, however, neuroimaging must be performed.
Neuroimaging of acute stroke
The brain should first be imaged with noncontrast computed tomography, which reliably distinguishes acute intracerebral hemorrhage (ICH) from ischemia [see Figure 2]. This distinction is critical because the management of hemorrhagic stroke [see Hemorrhagic Stroke, below] is substantially different from that of ischemic stroke. CT is relatively insensitive to ischemic changes in the first few hours after stroke. Early CT findings may be subtle [see Figure 2], but hyperdense vessels (suggestive of acute thrombus), loss of boundaries between gray and white matter, and effacement of cerebral sulci are highly specific for ischemic stroke.
The ability to identify at-risk cerebral tissue has considerable clinical relevance because therapy has the potential to salvage viable tissue.10 Physiologic imaging may detect the presence of so-called ischemic penumbra (tissue with impaired blood flow but active metabolism) surrounding infarcted tissue [see Figure 3]. Aerobic metabolism cannot be sustained in the face of inadequate blood flow, and necrosis ultimately results. The time course of this process is poorly defined, although substantial volumes of brain potentially remain viable for up to 17 hours after stroke. Diffusion-weighted imaging (DWI) reveals changes in infarcted tissue hours before conventional CT or magnetic resonance imaging. Hyperintensity on DWI is predictive of the extent of infarction, and quantitative analysis of the apparent diffusion coefficient may identify areas of cytotoxic edema, suggesting permanently infarcted tissue. Perfusion-weighted imaging (PWI) demonstrates regionally reduced cerebral blood flow. The difference between the perfusion defect and the diffusion defect may represent penumbral tissue, which is the target of acute therapy [see Figure 3]. Further, MRI spectroscopy may provide biochemical markers of the extent of ischemic injury. However, these MRI techniques are not available emergently in many centers, which limits their clinical utility at present.
Figure 1 Clinical pathway for acute stroke. (ABC—airway, breathing, and circulation; CBC—complete blood count; CT— computed tomography; ICH—intracranial hemorrhage; PT— prothrombin time; PTT—partial thromboplastin time; rt-PA— recombinant tissue plasminogen activator; SAH—subarachnoid hemorrhage)
Several imaging techniques may characterize the acute vascular lesion.10 The gold standard is conventional angiography, which may demonstrate acute occlusion or an embolus lodged at a vascular bifurcation. The vasculature can also be visualized quickly and noninvasively with CT angiography (CTA) and magnetic resonance angiography (MRA). Alternatively, trans-cranial Doppler ultrasonography can provide indirect evidence of major vascular occlusion and offers the advantage of realtime bedside monitoring in patients who receive thrombolytic therapy.11
Ischemic Stroke
Patients who present with neurologic dysfunction of sudden onset or who report neurologic signs and symptoms evolving over a few minutes to a few hours are most likely experiencing a stroke. In most of these patients, the stroke is ischemic rather than hemorrhagic [see Table 1].
Etiology and pathogenesis
Classically, ischemic strokes have been categorized as either thrombotic or embolic; clinically, however, strokes resulting from thrombosis may be indistinguishable from those caused by embolism. Ischemic strokes are more reliably categorized as resulting from cardioembolism, large-vessel atherothromboem-bolism, small-vessel occlusive disease, or other identified mechanism or as cryptogenic (idiopathic).12 The first three causes ac count for 70% to 90% of ischemic strokes. The latter two causes are pathophysiologically, diagnostically, and therapeutically distinct, and they are, therefore, are discussed separately [see Uncommon Causes of Ischemic Stroke, below].
Common Mechanisms
Cardioembolism Cardioembolism most commonly results from atrial fibrillation, mural thrombus, ventricular akinesis after myocardial infarction, dilated cardiomyopathy, and valvular disease [see Figure 4]. In each of these disorders, thrombus develops within the heart and embolizes to the brain. Ischemic events may be multiple and may occur in any major vessel. Therefore, cardioembolism must be considered in nearly every ischemic stroke patient.
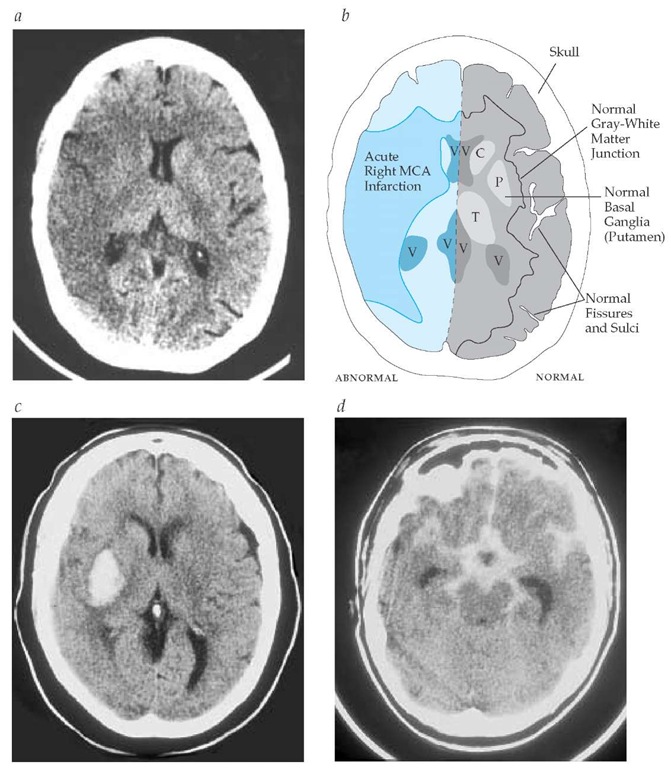
Figure 2 (a) Early computed tomographic findings in acute ischemic stroke. Three hours after onset of left hemiparesis and neglect, this noncontrast CT scan reveals extensive early findings in the right hemisphere, including obscuration of the gray-white junction and the basal ganglia and effacement of the cortical sulci. (b) Detail of CT findings shown in panel a. (c) Noncontrast head CT scan of a right putamenal intracerebral hemorrhage. (d) Noncontrast head CT scan of a subarachnoid hemorrhage manifesting the classic star-shaped area of hyperdensity in the basal cisterns. (C—caudate nucleus; P—putamen; T—thalamus; V—ventricles)
Figure 3 Magnetic resonance imaging scan showing perfusion-diffusion mismatch in acute ischemic stroke. The mismatch between diffusion and perfusion abnormalities may represent the ischemic penumbra (a). This patient presented with right hemiparesis, right-visual-field impairment, right sensory loss, and aphasia. The area of decreased perfusion (b) is much larger than the area identified on diffusion imaging (c), suggesting that the surrounding tissue may still be salvageable.
Large-vessel atherothromboembolism Atherosclerotic narrowing of the major extracranial or intracranial arteries may cause acute occlusion of the vessel itself, distal artery-to-artery embolic stroke, or focal hypoperfusion [see Figure 5]. A hallmark is the recurrence of similar clinical events, as a result of ischemia in the same vascular territory caused by involvement of a single large vessel. In general, atherosclerosis occurs at major arterial branches (e.g., the carotid bifurcation in the neck or intracranial branch points) and at vessel origins (e.g., the origin of the vertebral artery from the subclavian artery).
Small-vessel occlusive disease Small vessels are tiny terminal branches of larger vessels, such as those in the internal capsule, corona radiata, thalamus, and pons. Occlusion of these small vessels is often synonymous with so-called lacunar infarction. The mechanism of the occlusive process is uncertain, but lipohyalinosis, local atherosclerosis, and microthrombosis are possible. The process is most common in patients with longstanding diabetes or hypertension and is characterized by several specific clinical syndromes [see Clinical Manifestations and Lesion Localization, below]. The diagnosis of small-vessel disease rests on the clinical syndrome and the absence of an alternative etiology.