Cancer of the Kidney
Renal neoplasms are uncommon, accounting for 3% of all adult malignancies in Western society. The majority of these cancers are renal cell carcinomas (RCC), although other rare tumors of the kidney have been described, including transitional cell carcinomas, sarcomas, adult Wilms tumors, peripheral neuroecto-dermal tumors, lymphomas, and germ cell tumors.32,33 It should not be forgotten that tumors can metastasize to the kidney, commonly from lung, breast, and gastrointestinal tract malignancies. Transitional carcinomas occur predominantly in the renal pelvis and are managed in similar fashion to transitional cell carcinomas that arise in the bladder and other sites.
Epidemiology and etiology
RCC affects 30,600 people in the United States annually, and the incidence is steadily rising, with more than 10,000 related deaths occurring each year.34 This disease usually presents during the fifth to seventh decades of life, with a median age at diagnosis of 60 years. The incidence in men is twice that in women. RCC occurs in all ethnic groups, with no racial predilection. Most RCCs occur sporadically, but about 4% of cases present in an inherited pattern. Such familial renal cancers include von Hippel-Lindau (VHL) disease35 and familial papillary renal cell cancers. Approximately 1.6% of RCCs are part of the autosomal dominant VHL disease, which is also characterized by retinal and central nervous system hemangioblastoma, pheochromocy-toma, and pancreatic cyst. Compared with sporadic cases, RCCs in the VHL syndrome tend to be multifocal and bilateral and appear at a younger age. RCC and malignant angiomyolipoma are also associated with tuberous sclerosis complex, an autosomal dominant disorder of unknown etiology characterized by seizures, mental retardation, and hamartomas.
The true etiology of RCC remains largely undefined.34 Documented associations include smoking, diet, obesity, and hypertension. The relationship between RCC and the use of antihyper-tensive agents remains controversial. Patients with autosomal dominant or recessive polycystic kidney disease are not at higher risk than the general population. However a threefold to sixfold higher incidence of RCC has been found in patients undergoing long-term dialysis and in renal transplant recipients, presumably because of the development of acquired cystic kidney disease. Occupational exposure to chemicals appears to have little consistent significance, although a range of different occupations has been implicated in isolated case-control studies.
The isolation of the VHL gene in 1993 on chromosome 3p25 was a critical step in the understanding of the molecular genetics of renal cell carcinoma.35 Subsequently, the gene was found to encode for a tumor suppressor protein that prevents the formation of a transcriptional elongation complex and inhibits the transcription of certain target genes in cell proliferation. At the molecular level, both copies of the VHL gene must be inactivated for tumors to develop; therefore, the first mutation in the VHL gene is inherited in the germline, and inactivation of the remaining wild-type allele occurs as a somatic event. Inactivation of the VHL gene could also be responsible for the nonhereditary forms of RCC, given that somatic mutation of the VHL gene has been found in 75% to 80% of sporadic RCC cases. To date, the VHL gene does not appear to be involved in the development of papillary renal carcinomas.
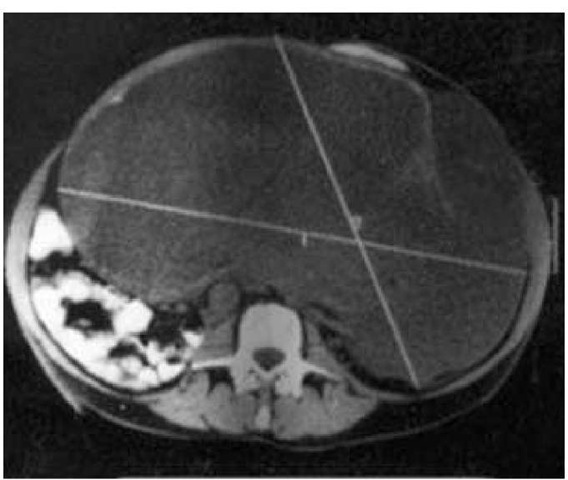
Figure 3 Renal cell carcinoma. Figure shows a huge primary renal cell carcinoma that occupied a substantial part of the abdominal cavity but that was surgically resected without tumor spillage.
Pathobiology
Recent immunohistochemical studies suggest that RCCs may arise from the proximal convoluted tubular cells. Grossly, the tumors are often well demarcated, round masses protruding from the cortex. RCCs of the clear cell type are usually yellow because of high lipid content. On cross section, areas of necrosis, cystic degeneration, hemorrhage, and calcification are commonly pre-sent.36 Histologically, RCCs are classified into five types: clear cell (75% to 85% of cases), chromophilic (12% to 14%), chromophobic (5%), oncocytic (2% to 4%), and collecting duct (1%).
Diagnosis
Clinical and Laboratory Findings
With the increasing application of ultrasound and CT scans to the diagnosis of nonspecific abdominal symptoms and other conditions, 20% to 30% of RCCs are discovered incidentally as small tumors in a clinically asymptomatic stage. The classic triad of hematuria, flank pain, and a palpable mass has become a less common presentation and usually portends an unfavorable out-come.37 However, presentation of one or two of these three symptoms is common: about 70% of patients have gross or microscopic hematuria, and 50% have abdominal or flank pain. By contrast, only 40% have a palpable mass. Constitutional symptoms (e.g., fever, night sweats, anorexia, and weight loss) are common. Exfoliative cytology of the urine may indicate the presence of carcinoma cells. Initial blood tests may reveal elevation of serum creatinine or uric acid levels; in more advanced disease, increased serum alkaline phosphatase concentrations or manifestations of paraneoplastic syndromes (see below) may be found.
Because the renal bed is a clinically silent area, some tumors are huge at first presentation [see Figure 3]. For the same reason, only 40% of patients have disease confined to the kidney at diagnosis, and nearly one quarter of patients present with symptoms of metastatic lesions. As the tumor grows, it may extend into and invade surrounding structures, such as the perinephric fat, posterior abdominal wall, inferior vena cava, ureter, adrenal gland, spleen, liver, or pancreas.38 Hypertension may develop if the tumor compresses the renal parenchymal vasculature, causing increased renin production. Metastatic spread occurs via either the blood or the lymphatics. Common sites of distant metastasis include the lungs, lymph nodes, liver, bone, and brain, although metastatic lesions have been described in virtually every organ of the body.
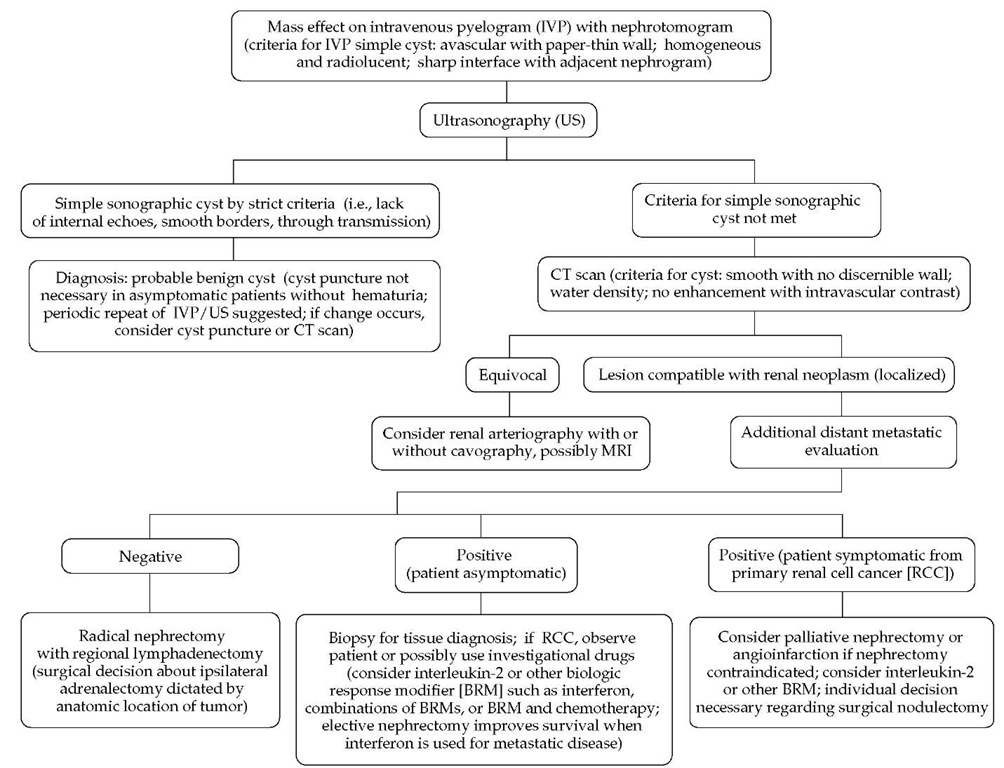
Figure 4 Algorithm for the diagnostic evaluation of a renal mass.
Paraneoplastic syndromes are relatively common features in RCC, the most notable ones being hypercalcemia and erythrocy-tosis—from the ectopic secretion of parathyroid hormone (PTH)-like immunoreactive protein and erythropoietin, respectively. Anemia is often present. Hyponatremia from inappropriate secretion of antidiuretic hormone (ADH) and Cushing syndrome from ectopic production of adrenocorticotropic hormone (ACTH) also occur occasionally. Hypercoagulable states presenting as venous thrombosis or pulmonary embolism have been described. Other rare paraneoplastic syndromes that have been reported include amyloidosis, nonmetastatic hepatic dysfunction, limbic encephalitis, erythema gyratum repens, myopa-thy, and polymyalgia rheumatica.
Imaging Studies
In a patient with symptoms or signs that suggest a renal malignancy, the initial demonstration of a mass lesion is usually achieved with an intravenous pyelogram (IVP) or ultrasonogra-phy [see Figure 4]. An IVP may differentiate between renal cysts and malignant lesions and may reveal complications such as ureteral obstruction and hydronephrosis. Ultrasonography is a convenient method of evaluating a suspicious renal mass and is especially useful in differentiating between solid lesions and cystic lesions. When combined with an IVP, ultrasonography differentiates benign cysts from solid masses with an accuracy of more than 90%. It can also be used to evaluate the size of the contralat-eral kidney and to rule out obstruction and hydronephrosis, but it is not reliable for assessment of the renal vein, intrahepatic inferior vena cava, and retroperitoneal lymph nodes.
CT scanning is the technique of choice for diagnosis and staging. Performed with intravenous contrast, CT accurately defines the renal topographic anatomy, the size and location of the tumor, and the relationship of tumor to the surrounding vessels (renal vein and inferior vena cava). It also evaluates possible ex-tracapsular spread, involvement of blood vessels or regional lymph nodes, and invasion of adjacent organs such as the adrenals, spleen, pancreas, and liver. However, CT is less sensitive in detecting lesions smaller than 2 cm or tumor extension to the perinephric fat. Furthermore, its utility is reduced in patients in renal failure because intravenous contrast cannot be used safely in such cases. MRI may be superior to CT in detecting tumors that are less than 2 cm in diameter, in detecting ex-tracapsular extension of tumor, and in delineating local anatomy. However, because of its expense and limited availability, MRI is often reserved for patients with known contraindications to iodine contrast or for when CT results are equivocal. The role of PET scanning has not been defined in this disease. Because 70% to 80% of RCCs are hypervascular, angiography is sometimes used to depict the lesion and provide a vascular map for the surgeon. Angiography is expensive and invasive and, therefore, should be used only for tumors in a solitary kidney, for vascular mapping before surgery, or for tumors in which an-gioinfarction is planned.
Staging
Staging of RCC is extremely important but sometimes difficult. The staging process should include a detailed history and physical examination, basic hematologic and biochemical studies, and a chest roentgenogram. A chest CT scan is sometimes necessary to rule out occult pulmonary and mediastinal metastasis. A bone scan should be obtained to rule out skeletal metastasis, although the yield is particularly low in asymptomatic patients with normal blood levels of alkaline phosphatase. Imaging of the brain with CT or MRI should be done in patients who have neurologic symptoms or signs. Fine-needle aspiration (FNA) of the tumor is usually not necessary, because if the FNA result is normal, the lesion will still need to be excised, given the possibility of a false negative result. On the other hand, if there is apparent extrarenal involvement of tumor, a CT-guided or ultrasound-guided biopsy of the primary lesion may be indicated if definitive surgical resection is not planned.
Tumor staging follows the standard TNM system and is predicated on the extent of the primary tumor (T), involvement of lymph nodes (N), and presence or absence of metastases (M).39 Traditionally, T1 tumors have been defined as those limited to the kidney, with a diameter of 7 cm or less; T2 tumors, those limited to the kidney but greater than 7 cm in diameter; T3 tumors, those extending into major veins or invading adrenal or peri-nephric tissues but not beyond the Gerota fascia; and T4 tumors, those extending beyond the Gerota fascia. A recent modification of the staging classification divides T1 tumors into T1a (tumors 4 cm or less in diameter) and T1b (tumors greater than 4 cm in diameter), on the basis of reports showing lower 5- to 10-year survival for patients with larger T1 tumors.39
The pathologic stage of RCC is the most important prognostic factor. Stage I or II (tumor confined within the kidney capsule) is associated with a 5-year survival of 50% to 85%; stage III (tumor invading the renal vein, inferior vena cava, or regional lymph nodes), with 15% to 35% survival; and stage IV (T4 or N2 or metastatic disease), with 0% to 15% survival. It is still generally held that asymptomatic patients have a better prognosis than those with significant tumor-related symptoms, and normal performance status (i.e., 0) is being incorporated into many current prognostic algorithms as a favorable determinant. Important adverse prognostic factors include microscopic vascular invasion, high nuclear grade, and aneuploid DNA content. Mathematical models are being developed to incorporate these prognostic determinants into a single, predictive algorithm for individual patients,40 but these algorithms have not yet been incorporated into standard practice internationally and still require extramural validation.
Non-clear cell histology has been investigated as a prognostic determinant but has proved difficult to assess because of the relatively small numbers of cases and the heterogeneity of histolog-ic types (including chromophobe, collecting duct, and papillary carcinomas). However, a recent study suggests that this histolog-ic grouping is associated with resistance to systemic therapy and poor survival, compared with the more common clear cell vari-ants.41 Similarly, Zisman and colleagues42 have assessed the prognostic implications of the so-called unclassified RCC variant, comparing 29 cases of unclassified histology against a series of 264 clear cell cancers. This study also showed a more aggressive biologic behavior and worse clinical outcome, but it did suggest that immunotherapy in addition to nephrectomy may lead to an improved outcome, compared with nephrectomy alone or no active treatment. By contrast, another variant of renal malignancy, the so-called oncocytoma, characterized by dense infiltration of mitochondria within the cytoplasm, rarely metastasizes, and outcome is excellent with surgical management alone.
Management
Stages I, II, and III Renal Cell Carcinoma
The standard treatment for RCC in stages I through III is surgical resection. The conventional procedure is radical nephrecto-my, which involves the en bloc removal of Gerota fascia with its contents and, usually, ipsilateral adrenalectomy.43 Regional lymph nodes are resected during surgery. However, in patients with bilateral tumors, tumors in a solitary kidney, or a poorly functioning contralateral kidney, nephron-sparing surgery (partial nephrectomy or enucleation) may be considered. Recent studies have shown that for tumors 4 cm or less in size, nephron-sparing surgery provides the same rate of survival as radical nephrectomy.4446 However, nephron-sparing surgery does carry some risk of postoperative complications, such as hemorrhage and fistula formation, so the selection of candidates is important. In some centers, laparoscopic nephrectomy is being performed routinely for lesions of 4 to 5 cm or less, irrespective of whether nephron preservation is planned. Proponents suggest that this approach produces less morbidity because the scar is smaller and there is less disruption of the abdominal contents. Caution must also be exercised in case selection, to reduce the risk of inadequate or incomplete tumor clearance. Preoperative or postoperative radiation therapy does not reduce local recurrence or increase survival.
Stage IV Renal Cell Carcinoma
The outcome for patients with metastatic stage IV RCC is generally very poor, because RCCs are usually resistant to chemotherapy.38 Most older clinical studies have shown response rates below 6%. Continuous infusion of floxuridine has reportedly produced response rates between 14% and 27%, although the responses are usually partial and short-lived. Investigators at the University of Chicago have published provocative data suggesting that the combination of weekly gemcitabine plus continuous-infusion fluorouracil (5-FU) may yield sustained remissions in up to 10% of cases,47 but these data remain to be confirmed. In many cases of reported remission, the existence of true metastatic disease had not been confirmed histolog-ically before the use of chemotherapy. This is important, because granulomatous reactions may be found in the lungs and lymph nodes of patients with purportedly metastatic RCCs.
Surgical intervention is recommended in patients with solitary metastatic lesions that are resectable. Radiation therapy can be used to palliate symptoms from metastases to the bone or brain or from those causing spinal cord compression.
Spontaneous regression of metastatic RCC lesions has been reported in the literature, with an incidence rate of 0.8% to 3%. This has been reported for metastatic sites, including the brain, bone, regional and distant lymph nodes, liver, and, most commonly, the lungs. Complete and durable disappearance of tumors has been documented. The mechanism for this phenomenon is unknown, but it has been attributed to immunologic factors associated with reduction of tumor bulk. In many instances, spontaneous regression has occurred in supposed metastases that have not been confirmed by biopsy, and it is possible that these may have been reactive granulomata. Many of the so-called spontaneous regressions have followed surgical removal of the primary tumor, but in view of the relative rarity of this phenomenon, nephrectomy should not be routinely recommended in patients with stage IV RCC for the sole purpose of achieving spontaneous regression. Rather, nephrectomy should be considered for palliation of local symptoms such as pain and hematuria.
In a recent multicenter study of patients with advanced RCC, nephrectomy plus systemic immunotherapy with interferon was found to provide a statistically significant survival benefit over immunotherapy alone (12 months versus 8.5 months).48 Although this difference is not very large, the order of magnitude is similar to the difference between MVAC and single-agent cis-platin in the Intergroup Advanced Bladder Cancer Study,25 a trial that led to the establishment of MVAC as a standard of care. In addition, the tails of the survival curves were statistically different. However, critics have suggested that the results of the nephrectomy-plus-immunotherapy arm are equivalent to those reported in trials of single-modality therapy in Europe and that caution should be used in interpreting these data at this early stage. Such criticism is reasonable, but the fact is that this was a well-constructed, randomized trial with a comparison reflecting randomized cohorts. As such, this trial was less likely to be subject to the various selection biases of historical or nonrandom-ized comparisons. Consequently, it seems reasonable to view the combination of nephrectomy and immunotherapy as a new standard of care. However, it is not yet clear whether this applies to combinations that involve interleukin-2 (IL-2) and other, more novel cytokines.
IL-2 works through the activation of cytotoxic T cell subgroups and stimulation of cytokine release. A multicenter trial of IL-2 in patients with metastatic RCC showed therapeutic benefit (complete response rate, 4%; partial response rate, 8%; median duration of response, 23 months) but also displayed substantial toxici-ty, mainly from a capillary leak syndrome, which can cause cardiovascular ischemia, renal failure, and shock. Supportive measures such as use of vasopressors and intensive care unit monitoring are often needed.49 Interferon alfa has been reported to be effective, with an overall response rate of 12%, although complete responses are less common.50 A recent randomized study comparing IL-2 alone, interferon alfa alone, and the two agents together found a significantly higher response rate in the combination group (18.6%, versus 6.5% for IL-2 and 7.5% for interferon alfa).50 Event-free survival at 1 year was also higher in the combination group, although there was no difference in overall survival.50 However, other studies using similar combinations have failed to show any benefit over IL-2 used alone.51 In general, it is believed that improved outcomes appear to be associated with the use of cytokines,52 although most of the available data appear to be subject to the limitations of major case selection bias.
Immunologic factors seem to influence long-term survival in patients with advanced RCC. On the basis of studies showing that allogeneic stem-cell transplantation can induce curative graft-versus-leukemia reactions in some hematologic malignancies, transplantation of nonmyeloablative allogeneic peripheral blood stem cells has been used in advanced RCC, with some suc-cess.53 Very heavy case selection occurs in the application of such strategies, however, and graft versus host disease (GVHD) is a very significant problem.