The complete evaluation of the cardiovascular patient begins with a thorough history and a detailed physical examination. These two initial steps will often lead to the correct diagnosis and assist in excluding life-threatening conditions. The history and physical examination findings should be assessed in the context of the overall clinical status of the patient, including lifestyle, comorbidities, and expectations. Cardiovascular conditions that frequently require evaluation include chest pain, dyspnea, palpitations, syncope, claudication, and cardiac murmurs. Each of these conditions will be discussed separately, with an emphasis on a diagnostic algorithm and the appropriate use of invasive and noninvasive cardiac testing.
Chest Pain
Bckground
Chest pain is perhaps the most common cardiovascular symptom encountered in clinical practice. Establishing a cardiac origin of chest pain in a patient with multiple cardiovascular risk factors is essential because it allows initiation of appropriate therapy, thereby reducing the risk of myocardial infarction and death. Similarly, excluding a cardiac origin of chest pain in a low-risk patient is no less essential to avoid costly and potentially risky diagnostic testing that will neither add to the care of the patient nor relieve the patient’s discomfort.1 Cardiac disorders that result in chest pain include myocardial ischemia, myocar-dial infarction, acute pericarditis, aortic stenosis, hypertrophic cardiomyopathy, and aortic dissection. Noncardiac disorders that may result in chest pain include pulmonary embolism, pneumonia, pleural effusion, reactive airway disease, gastrointestinal and biliary disease, anxiety, and musculoskeletal disorders.
Angina most frequently is caused by atherosclerosis of the coronary arteries. Less common causes of angina include coronary artery spasm (e.g., Prinzmetal angina or spasm secondary to drug use, as with cocaine), coronary artery embolism (from aortic valve endocarditis), congenital coronary anomalies, spontaneous coronary artery dissection, coronary arteritis, and aortic dissection when the right coronary artery is involved. Angina may also occur in the presence of angiographically normal coronary arteries and is referred to as syndrome X. The underlying pathophysiology is thought to be related to microvascular dysfunction; the prognosis is generally good despite frequent episodes of chest pain.
History and physical examination
Essential features of the history include an accurate description of the chest pain, including the severity, frequency, location, radiation, quality, alleviating and aggravating factors, and duration of symptoms [see Table 1]. Anginal chest pain is often described as pressure or a heavy sensation. Symptoms may be difficult for the patient to describe and may be better characterized as discomfort, not pain. Angina typically is described as subster-nal with radiation to the left neck, jaw, or arm; is mild to moderate in severity; and lasts for 5 to 15 minutes. Classically, angina occurs with exercise, stress, or exposure to cold weather and is relieved with rest or use of nitroglycerin. Some of the most useful features of the patient history that help establish that chest pain is angina are (1) reproducibility of the pain with a given degree of activity, (2) brief duration, and (3) alleviation of the pain with rest or use of nitroglycerin. In patients with a history of coronary artery disease (CAD), an accurate characterization of the quality and frequency of the pain is essential to determine whether a change in the anginal pattern has occurred (i.e., a patient with chronic stable angina now has unstable angina) or if a noncardiac origin of pain is now present (e.g., a patient with chronic stable angina now has musculoskeletal pain). Elderly patients, diabetic patients, and women experiencing angina often present with atypical symptoms that may appear to be non-cardiac in nature.
Anginal chest pain may also be seen in patients with aortic stenosis or hypertrophic cardiomyopathy secondary to the supply-demand imbalance caused by excessive myocardial hypertrophy. Pericarditis commonly results in a sharp type of chest pain that occurs in the substernal region and worsens on inspiration (pleuritic) when the patient is in a supine position and improves when the patient is in an upright position. The pain of aortic dissection is also substernal, but typically, it is described as a tearing or ripping sensation, radiates to the back or inter-scapular area, begins abruptly, and fails to improve with rest or use of nitroglycerin. Musculoskeletal pain may be located anywhere on the chest wall, is often reproducible with palpation, and frequently worsens with rotation of the thorax. If the pain is musculoskeletal in origin, recent episodes of excessive lifting or activity may be elicited in the history. Esophageal spasm and gastroesophageal reflux disease are frequent causes of noncar-diac chest pain.3
Cardiovascular risk factors should be reviewed in all patients presenting with chest pain. These risk factors include (1) a history of hypertension, hyperlipidemia, diabetes mellitus, or cigarette smoking,4 and (2) a family history of CAD (i.e., a first-degree male relative with myocardial infarction or sudden death occurring before 55 years of age or a first-degree female relative with these events occurring before 65 years of age). Relatively uncommon factors that may also result in angina include prior radiation therapy, drug use (e.g., cocaine and amphetamines), and the presence of a systemic disease (e.g., lupus erythemato-sus, polyarteritis nodosum, or rheumatoid arthritis) that is associated with coronary arteritis.
The physical examination is usually unremarkable in patients presenting with anginal chest pain. However, certain physical findings can be very helpful in supporting the diagnosis of CAD. Elevated blood pressure by cuff sphygmomanometry and retinal abnormalities on fundoscopic examination (e.g., arterio-venous nicking, microaneurysms, arteriolar narrowing, or hemorrhages) may indicate previously undiagnosed hypertension. Xanthomas (cholesterol-filled nodules that occur subcutaneous-ly or over tendons) indicate severe elevations in serum cholesterol levels. Femoral, carotid, or renal artery bruits and diminished peripheral pulses signify peripheral vascular disease and markedly increase the probability of CAD.5 Tenderness to palpation of the chest wall, especially at the costochondral and chondrosternal articulations, suggests a musculoskeletal etiology of chest pain. Occasionally, patients with anginal chest pain also have a component of reproducible pain with palpation. A third heart sound and a holosystolic murmur of mitral regurgi-tation (secondary to ischemia of a papillary muscle) may be present if a patient with CAD is examined during an episode of anginal pain.
Physical examination also is directed toward findings that suggest an alternative cause of chest pain. Asymmetrical peripheral pulses, an early diastolic murmur, and the appropriate clinical history (tearing chest pain with radiation to the back) indicate an aortic dissection. A systolic murmur that radiates to the base of the neck (aortic stenosis) or a systolic murmur that increases in intensity with the strain phase of the Valsalva maneuver (hypertrophic cardiomyopathy) are uncommon but useful findings. A so-called leatherlike or scratchy series of sounds indicates a pericardial rub and supports a diagnosis of pericarditis. The intensity of the rub may increase with inspiration, indicating associated inflammation of the pleura, or pleuritis. Examination of the lung fields may disclose diminished breath sounds associated with dullness to percussion (pleural effusion), rhonchi, and egophony (pneumonia) or expiratory wheezes (asthma).
Diagnostic tests
On the basis of the history, chest pain is characterized as anginal, atypical anginal (some features of angina combined with some noncharacteristic features), or nonanginal. Estimates of the pretest probability of CAD can be accurately derived from a description of the chest pain syndrome and the presence or absence of cardiovascular risk factors.6,7 The most widely used method for determining pretest likelihood of CAD is the Duke University Database formula, which considers the patient’s age, sex, cardiovascular risk factor profile, description of chest pain, and information from the resting electrocardiogram.8
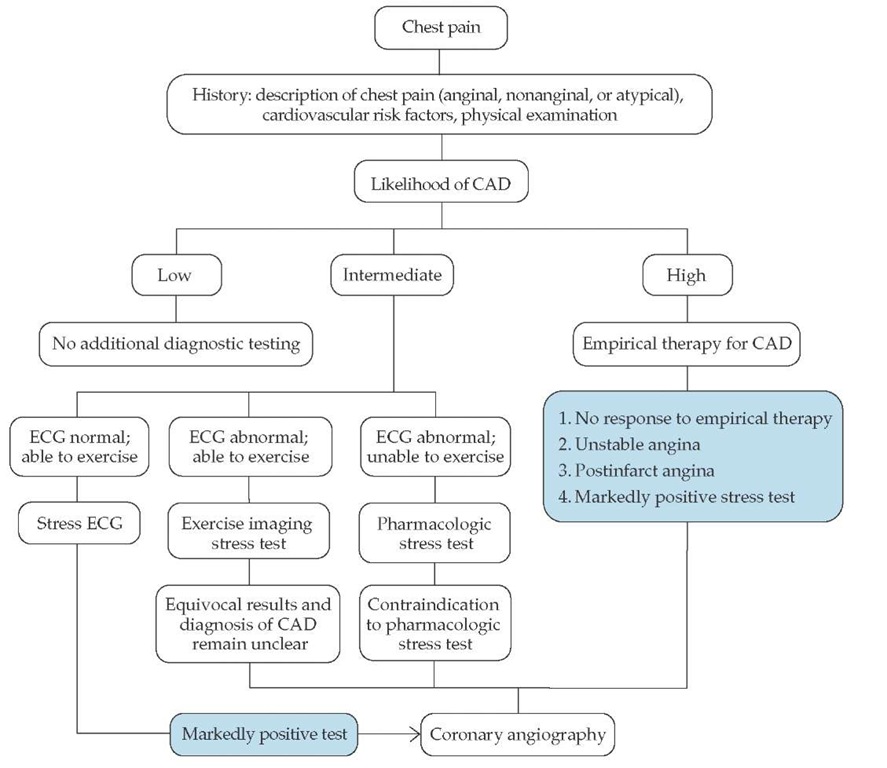
Although the diagnostic yield from the baseline ECG is low, it provides useful information on the advisability of pursuing additional diagnostic testing [see Figure 1]. Notable findings include Q waves consistent with a prior myocardial infarction and left ventricular hypertrophy that may be secondary to aortic stenosis, hypertrophic cardiomyopathy, or long-standing hypertension. ST segment depression, T wave abnormalities, and arrhythmias may be present if the ECG is obtained during an episode of anginal chest pain. A normal resting ECG predicts normal left ventricular function with a high degree of certainty (i.e., > 95%).
As with the ECG, a routine chest roentgenogram is usually normal. However, the presence of cardiomegaly, a left ventricular aneurysm, significant coronary or aortic calcification, or pulmonary venous congestion would be useful information and may warrant additional diagnostic testing.
Some physicians have started to use portable or handheld echocardiographic devices to evaluate patients with chest pain. Pertinent findings by echocardiography that would assist in establishing the etiology of chest pain include a pericardial effusion (pericarditis), hypokinesis or akinesis of a left ventricular wall segment (acute coronary ischemia), a dilated right ventricle (pulmonary embolism), and calcification and impaired excursion of the aortic valve leaflets (aortic stenosis).
Noninvasive stress testing is most likely to influence clinical decision making when the pretest probability of CAD is in the intermediate range. Patients with a low risk of CAD should not undergo noninvasive cardiac stress testing, because an abnormal test result would likely be a false positive one, and a negative test result would simply confirm the low probability of CAD. However, if patient reassurance is a consideration, a normal test result may be very useful. In addition, exercise stress testing provides information regarding symptom status, exercise capacity, and the hemodynamic response to exercise if the history is unclear (e.g., the patient denies symptoms but has decreased exercise capacity for "other reasons"). Absolute and relative contraindications to exercise testing should be reviewed in all patients before testing is begun [see Table 2].8
Similarly, patients with a high risk of CAD in general should not undergo noninvasive cardiac stress testing for the purpose of diagnosing CAD, because a negative test result would likely be a false negative one, and a positive result would simply confirm the high probability of CAD. In such patients, coronary an-giography should be used to establish a diagnosis of CAD. However, noninvasive cardiac stress testing in certain patients at high risk for CAD may be useful. Indications for noninvasive stress testing in these patients include (1) assessment of the effectiveness of current medical therapy, (2) objective measurement of exercise capacity, (3) evaluation of the extent and location of ischemia or infarction with nuclear or echocardiographic imaging, (4) preoperative risk assessment in patients with known CAD who are undergoing noncardiac surgery, and (5) assessment of prognosis in patients with symptoms consistent with CAD or in patients with known CAD.
Table 1 Differentiating Features in the Patient’s History of Chest Pain
|
Condition |
Location |
Radiation |
Quality |
Alleviating Factors |
Aggravating Factors |
Duration |
|
Angina pectoris |
Substernal |
Jaw, arm |
Pressure |
Rest, nitroglycerin |
Exercise, cold weather |
5-15 minutes |
|
Pericarditis |
Left-sided, substernal |
Neck, trapezius ridge |
Sharp |
Sitting up and leaning forward |
Inspiration, supine position |
Hours |
|
Musculoskeletal |
Variable over entire chest wall |
None |
Sharp or aching |
Rest, anti-inflammatory or analgesic medications |
Movement, palpation |
Variable, but usually constant |
|
Aortic stenosis |
Substernal |
Occasionally to jaw, arm |
Pressure |
Rest, nitroglycerin |
Exercise, cold weather |
Minutes |
|
Hypertrophic cardiomyopathy |
Substernal |
Occasionally to jaw, arm |
Pressure |
Rest, nitroglycerin |
Exercise, cold weather |
Minutes |
|
Aortic dissection |
Substernal |
Back |
Tearing |
None |
None |
Minutes to hours |
Figure 1 Evaluation of patients with chest pain. (CAD—coronary artery disease; ECG—electrocardiogram)
To establish the diagnosis of CAD in intermediate-risk patients, a number of noninvasive testing methods are available.9 The decision whether to perform a specific test is based on various patient characteristics (e.g., body size, associated medical conditions, and ability to exercise), findings on the baseline ECG, and institutional experience with specific testing methods [see Table 3].10-16 The most appropriate noninvasive stress test is chosen on the basis of each of these factors, as indicated in the chest pain algorithm [see Figure 1]. For most patients who are able to exercise with a normal baseline ECG, treadmill-ECG stress testing is indicated [see Table 2].14,15,17 Women have a higher incidence of false positive results; therefore, many physicians recommend that, for all women, exercise be combined with an imaging method (e.g., echocardiography or nuclear imaging).11 In general, to establish the diagnosis of CAD, exercise is preferred over pharmacologic stress agents. For patients who are unable to exercise because of physical limitations (e.g., arthritis or orthopedic problems), severe coexisting pulmonary disease, or general disability, pharmacologic stress agents such as dobu-tamine, adenosine, or dipyridamole can be employed. Each of these agents has specific contraindications [see Table 4].
Coronary angiography is considered the gold standard for the diagnosis of CAD. Although the incidence of major compli cations is low (< 2%), coronary angiography is costly and has some risk; thus, it is reserved for (1) patients with markedly positive noninvasive tests (i.e., hypotension and significant ST segment depression on ECG stress testing on a treadmill), (2) patients at high risk for CAD in whom a course of empirical an-tianginal therapy has failed, (3) patients with unstable or postinfarction angina, (4) patients with a contraindication to ex-ercise or pharmacologic stress testing, and (5) patients with equivocal results on noninvasive stress testing when the diagnosis of CAD remains unclear.
Table 2 Absolute and Relative Contraindications to Exercise Testing8
|
Absolute |
Relative |
|
Recent myocardial infarction (within 48 hr) |
Left main coronary stenosis |
|
Unstable angina not previously stabilized with medical therapy |
Moderate stenotic valvular heart disease |
|
Uncontrolled cardiac arrhythmias causing symptoms or hemody-namic compromise |
Electrolyte abnormalities |
|
Symptomatic severe aortic stenosis |
Severe arterial hypertension |
|
Uncontrolled symptomatic heart failure |
Tachycardia or bradyarrhythmias |
|
Acute pulmonary embolism or pulmonary infarction |
Hypertrophic cardiomyopathy and other forms of outflow tract obstruction |
|
Acute myocarditis or pericarditis |
Mental or physical impairment leading to inability to exercise adequately |
|
Acute aortic dissection |
High degree of atrioventricular block |
Table 3 Diagnostic Testing Methods Available for Evaluating Chest Pain10-17
|
Diagnostic Test |
Indications |
Information Obtained |
Limitations |
Sensitivity |
Specificity |
|
Exercise electrocardiographic stress test (stress ECG) |
Initial test for most males with chest pain to establish diagnosis of CAD; females have higher rate of false positive test results Assess prognosis and functional capacity in patients with prior MI or known CAD Assess efficacy of current medical therapy in patients with known CAD |
Exercise duration and functional aerobic capacity Amount of ST segment depression as indication of extent of ischemia Hemodynamic response to exercise |
Normal baseline ECG Ability to exercise (patients who cannot attain adequate cardiopulmonary stress because of respiratory or musculoskeletal problems should receive a phar-macologic stress agent) Contraindications [see Table 2] False positives occur with left ventricular hypertrophy, bundle branch block, preexcitation syndromes, electrolyte abnormalities, and digoxin use |
68%1 (females, 61%2) |
77%1 (females, 70%2) |
|
Thallium-201 perfusion scintigraphy |
Often used when increased diagnostic accuracy for CAD required Can be combined with pharmacologic stress agents such as dobuta-mine, adenosine, or dipyridamole |
Diagnosis of CAD with higher sensitivity and specificity than stress ECG Extent of ischemia Extent of infarction Left ventricular cavity size |
Higher cost and longer testing time than stress ECG Imaging artifacts (attenuation) from diaphragm, breast, and intestine Contraindications [see Table 2 if exercise; see Table 4 if pharmacologic stress agent] |
Ex thall 89%5 Ph thall 90%5 Dob thall 88%4 |
Ex thall 76%5 Ph thall 70%5 Dob thall 74%4 |
|
Technetium-99m perfusion scintigraphy |
Often used when increased diagnostic accuracy for CAD required Can be combined with pharmacologic stress agents such as dobuta-mine, adenosine, or dipyridamole |
Higher sensitivity and specificity for diagnosis of CAD than stress-ECG Extent of ischemia Extent of infarction Left ventricular cavity size ECG-gated SPECT allows calculation of left ventricular ejection fraction and evaluation of wall motion; evaluation of wall motion reduces false positive scans caused by imaging artifacts (attenuation) Used when excessive body weight precludes thallium imaging |
Higher cost and longer testing time than stress ECG Imaging artifacts (attenuation) from diaphragm, breast, and intestine Contraindications [see Table 2 if exercise; see Table 4 if pharmacologic stress agent] |
Ex tech 89%5 Ph tech 90%5 Dob tech 88%4 |
Ex tech 76%5 Ph tech 70%5 Dob tech 74%4 |
|
Exercise or dobutamine echocardiography |
Exercise echocardiogra-phy often used when patient can exercise and has good-quality echocardiographic images Dobutamine used when exercise not possible |
Higher sensitivity and specificity for diagnosis of CAD than stress ECG Left and right ventricular chamber size and function, presence of valve disease and pulmonary arterial pressures |
Inadequate image quality may occur in patients with obesity, chronic obstructive pulmonary disease, and chest wall deformities Contraindications [see Table 2 if exercise; see Table 4 if pharmacologic stress agent] |
Ex echo 85%6 Dob echo 82%6 |
Ex echo 86%6 Dob echo 82%6 |
|
Holter monitoring |
Prinzmetal angina |
Transient ST segment elevation in presence or absence of chest pain |
Difficult to interpret because of baseline abnormalities |
||
|
Coronary angiography |
Chest pain of unclear etiology despite noninvasive testing Angina not responsive to medical therapy Unstable and postinfarc- tion angina Unclear diagnosis of CAD despite noninva-sive stress testing |
Anatomic severity of CAD Completely exclude cardiac origin of chest pain—gold standard of diagnostic tests Left ventricular function if left ventricular angiogra-phy also performed |
Invasive procedure with low (< 2%) but inherent risk of MI, stroke, and death Represents a luminogram; does not evaluate functional significance of arterial narrowing |
100% |
100% |
CAD—coronary artery disease
Dob tech—dobutamine technetium
Dob thall—dobutamine thallium
ECG—electrocardiogram
Ex echo—exercise echocardiography
Ex tech—exercise technetium
Ex thall—exercise thallium
MI—myocardial infarction
Ph stress—pharmacologic stress
Ph tech—pharmacologic (adenosine or dipyridamole) stress combined with technetium
Ph thall—pharmacologic (adenosine or dipyridamole) stress combined with thallium
SPECT—single-photon emission computed tomography
Table 4 Mechanism of Action, Side Effects, and Contraindications of Pharmacologic Stress Agents
|
Pharmacologic Stress Agent |
Mechanism of Action |
Side Effects |
Contraindications |
|
Dobutamine |
Increase myocardial oxygen demand by increasing heart rate, blood pressure, and myocardial contractility |
78% of patients experience side effects: chest pain, palpitations, headache, flushing, malaise, and dyspnea; ventricular and atrial arrhythmias may occur |
Severe hypertension at baseline, recent history of ventricular and/or atrial arrhythmias, and current beta-blocker use |
|
Dipyridamole |
Coronary artery vasodilatation— indirect response by blocking adenosine uptake and degradation |
Increase in heart rate (average, 5-10 beats a minute), decrease in systolic blood pressure (average, 10-15 mm Hg); approximately 50% of patients experience side effects: chest pain, flushing, dizziness, headaches, or nausea; may provoke bronchospasm |
Severe reactive airway disease (not con-traindicated with chronic obstructive pulmonary disease unless a significant component of reactive airway disease is present), current theophylline use; avoid caffeine use 1 day before testing |
|
Adenosine |
Coronary artery vasodilatation— direct response |
79% of patients experience side effects (more than with dipyridamole); side effects are chest, throat or jaw pain, headache, flushing, malaise, nausea, and bradyarrhythmias |
Similar to dipyridamole; avoid caffeine use 1 day before testing; may cause bradyar-rhythmias and is therefore contraindicated with baseline second- or third-degree heart block |
Coronary angiography has certain limitations, including the inability to determine (1) the functional significance of a coronary artery stenosis and (2) which coronary plaque is likely to rupture (i.e., the so-called vulnerable plaque) and result in an acute coronary syndrome. Intravascular ultrasound studies have shown that coronary angiography may occasionally underestimate the severity of an area of narrowing, because it represents a so-called luminogram (shadow image) and not the size of the atherosclerotic plaque.18 Despite these shortcomings, the extent and severity of CAD and measurement of left ventricular function by left heart catheterization are powerful predictors of clinical outcome.14