Hematology deals with the normal functions and disorders of the formed elements in the blood (i.e., erythrocytes, leukocytes, and platelets) and the plasma factors governing hemostasis. The blood sustains life by transporting oxygen and essential nutrients, removing waste, and delivering the humoral and cellular factors necessary for host defenses. Platelets and coagulation factors, together with vascular endothelial cells, maintain the integrity of this system. Some hematologic disorders such as anemia, leukocytosis, and bleeding are quite common, occurring secondary to infectious, inflammatory, nutritional, and malignant diseases. Other disorders, including the hemato-logic malignancies, are far less common. This subsection presents the general principles for understanding the hematopoiet-ic system [see other subsections under Hematology for a more detailed description of the pathophysiology of specific hematologic diseases and their treatment].
Hematopoiesis
Hematopoiesis begins in the fetal yolk sac and later occurs predominantly in the liver and the spleen.1 Recent studies demonstrate that islands of hematopoiesis develop in these tissues from hemangioblasts, which are the common progenitors for both hematopoietic and endothelial cells.2 These islands then involute as the marrow becomes the primary site for blood cell formation by the seventh month of fetal development.3 Barring serious damage, such as that which occurs with myelofibrosis or radiation injury, the bone marrow remains the site of blood cell formation throughout the rest of life. In childhood, there is active hematopoiesis in the marrow spaces of the central axial skeleton (i.e., the ribs, vertebrae, and pelvis) and the extremities, extending to the wrists, ankles, and the calvaria. With normal growth and development, hematopoiesis gradually withdraws from the periphery. This change is reversible, however; distal marrow extension can result from intensive stimulation, as occurs with severe hemolytic anemias, long-term administration of hematopoietic growth factors, and hematologic malignancies. The term medullary hematopoiesis refers to the production of blood cells in the bone marrow; the term extramedullary hematopoiesis indicates blood cell production outside the marrow in the spleen, liver, and other locations.
Organization of hematopoietic tissues
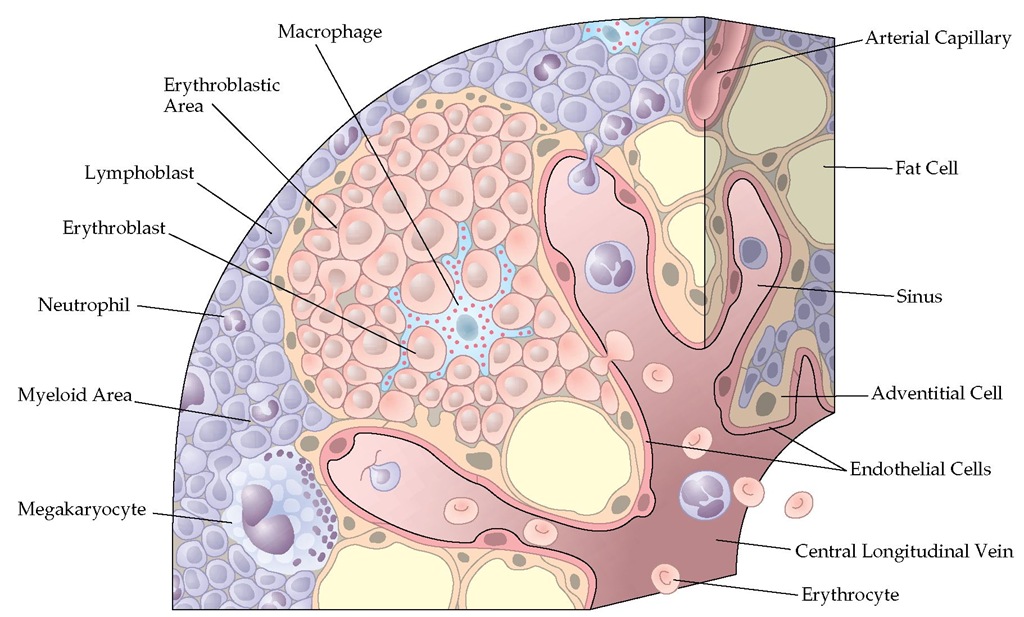
In its normal state, the medullary space in which hematopoi-etic cells develop contains many adipocytes and has a rich vascular supply [see Figure 1].4 Vascular endothelial cells, marrow fibroblasts, and stromal cells are important sources of the matrix proteins that provide structure to the marrow space; these cells also produce the hematopoietic growth factors and chemokines that regulate blood cell production.5 The vascular endothelial cells also form an important barrier that keeps immature cells in the marrow and permits mature hematopoietic elements to enter the blood. The abundant adipocytes may influence hematopoiesis by serving as a localized energy source, by synthesizing growth factors, and by affecting the metabolism of androgens and estrogens.6 Marrow macrophages remove effete or apoptotic cells and clear the blood of foreign materials when they enter the marrow. Osteoblasts and osteoclasts maintain and remodel the surrounding cancellous bone and the calcified lattice, which crisscrosses the marrow space.
The thymus, lymph nodes, mucosa-associated lymphatic tissues, and the spleen have multiple hematopoietic functions. Early in development, they are major sites of hematopoiesis. In adulthood, they are principally sites of lymphocyte development, processing of antigens, development of effector T cells, and antibody production [see 6 Immunology/Allergy]. In leukemia and the myeloproliferative disorders, the size and cellular architecture of these tissues are deranged, leading to many of the clinical manifestations of these disorders [see 12:XVI Acute Leukemia and 12:XVII Chronic Myelogenous Leukemia and Other Myeloproliferative Disorders].
Hematopoietic Stem Cells
All cells of the hematopoietic system are derived from common precursor cells, the hematopoietic stem cells.7 These cells are difficult to identify, in part because they normally represent only about 0.05% of marrow cells. Through self-renewal, this population is maintained at a constant level.8 Through the use of monoclonal antibodies that recognize specific cell surface molecules expressed selectively on developing hematopoietic cells and other specialized techniques, the stem cells can now be separated from other marrow cells. With these methods, very primitive hematopoietic stem cells have been found to be positive for c-kit and thy-1 but negative for CD34, CD38, CD33, and HLA-DR.8 For clinical purposes, CD34+ progenitor cell populations, which contain stem cells and some more mature cells, are often used for hematopoietic stem cell transplantation9 [see 5:XIHematopoietic Stem Cell Transplantation].
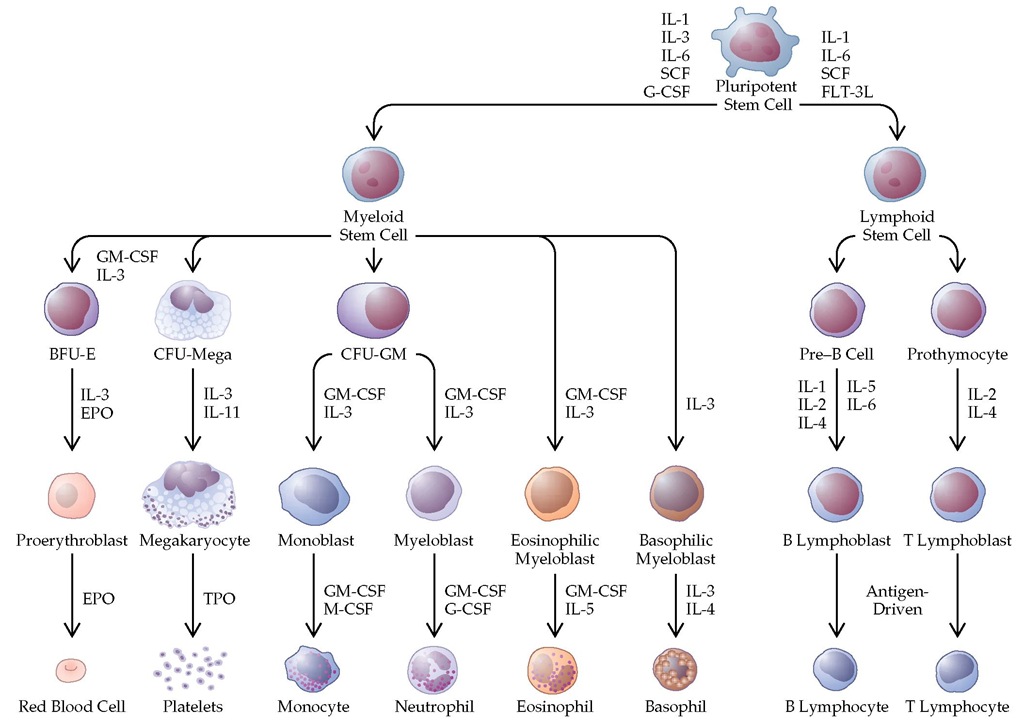
Stem cells give rise to daughter cells, which undergo irreversible differentiation along various hematopoietic cell lineages [see Figure 2].10 Many aspects of the earliest steps in this differentiation process are not well understood. With lineage commitment, however, differentiation, maturation, and release of cells to the blood come under the control of well-defined hematopoietic growth factors. In the early phases of differentiation, the regulatory roles played by these growth factors over-lap.11 Later in development, some growth factors are lineage specific, meaning that they govern the maturation and deployment of single lineages. Erythropoietin (EPO) (erythrocytes), thrombopoietin (TPO) (platelets), granulocyte colony-stimulating factor (G-CSF) (neutrophils), and macrophage colony-stimulating factor (M-CSF) (monocytes) are the best-characterized lineage-specific factors.
Hematopoietic Growth Factors
The hematopoietic growth factors, also referred to as hematopoietic cytokines, are a family of glycoproteins produced in the bone marrow by endothelial cells, stromal cells, fi-broblasts, macrophages, and lymphocytes; they are also produced at distant sites, from which they are transported to the marrow through the blood [see Table 1]. The naming of these factors is somewhat confusing. Erythropoietin and thrombopoietin derive part of their names from the Greek word poiesis, meaning "to make." The colony-stimulating factors were first recognized because of their capacity to stimulate early hematopoietic cells to grow into clusters and large colonies in tissue culture systems. The term interleukin denotes factors that are produced by leukocytes and that affect other leukocytes. This is a large family of factors that predominantly govern lym-phocytopoiesis, but many members also have broad effects on other lineages. The discovery of new growth factors and of the biologic consequences of deficiencies or excesses of these factors continues to evolve rapidly.
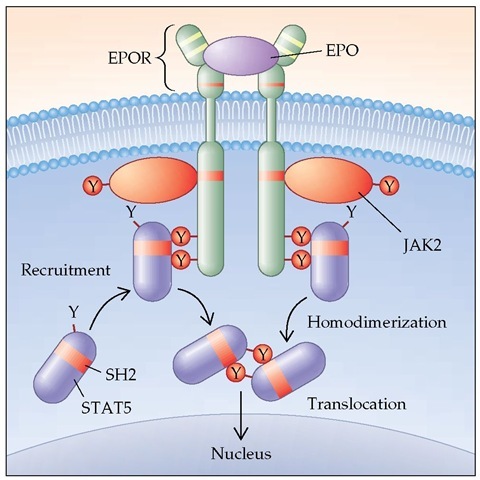
Hematopoietic cells have distinctive patterns of expression of growth factor receptors, and the patterns evolve as the cells differentiate [see Figure 2].11 Each growth factor binds only to its specific receptor.12 It is now known that some growth factors share components of the receptor (e.g., interleukin-3 [IL-3], IL-5, and granulocyte-macrophage colony-stimulating factor [GM-CSF] share a common | chain of their receptor); specificity comes from other unique or private components of the receptor. Binding of the ligand to the receptor leads to a conforma-tional change, activation of intracellular kinases, and, ultimately, the triggering of cell proliferation.13,14 For some growth factors, these pathways are well defined; for others, the pathways are still unclear [see Figure 3].
Hematopoietic growth factors not only stimulate cell proliferation but also prolong cell survival; that is, they have anti-apoptotic effects.15 For some lineages, such as neutrophils and monocytes, growth factor receptors occur on fully mature cells; exposure of these cells to the factors primes the cells for an enhanced responsiveness to bacteria or other stimulators of their metabolic activity. Thus, for cells of the neutrophil lineage, the growth factors G-CSF and GM-CSF can stimulate early hematopoietic cell proliferation, increase the number of cells produced by the marrow, prolong the life span of these cells, and augment cell functions.
Erythropoietin
The peritubular interstitial cells located in the inner cortex and outer medulla of the kidney are the primary site for erythropoi-etin production.17 In response to hypoxia, transcription of the ery-thropoietin gene in these cells increases, resulting in increased secretion of erythropoietin. The protein is then transported through the blood to the marrow to stimulate erythropoiesis. With renal failure, erythropoietin production is severely impaired. In infections and many chronic inflammatory conditions, the erythro-poietin response is blunted, and erythropoietin levels are low.18
Erythropoietin is a glycosylated protein that modulates erythropoiesis by affecting several steps in red cell development. The most primitive identifiable erythroid cells, the burst-forming unit-erythroid cells (BFU-E), are relatively insensitive to erythropoietin. More mature cells, the colony-forming unit-erythroid cells (CFU-E), are very sensitive. Erythropoietin treatment prolongs survival of erythroid precursors, shortens the time between cell divisions, and increases the number of cells produced from individual precursors.
Erythropoietin can be administered intravenously or subcu-taneously for the treatment of anemia caused by inadequate endogenous production of erythropoietin.20 Treatment is maximally effective when the marrow has a generous supply of iron and other nutrients, such as cobalamin and folic acid.21 For patients with renal failure, who have very low erythropoietin levels, the starting dosage is 50 to 100 units S.C. three times a week. The most easily monitored immediate effect of increased endogenous or exogenous erythropoietin is an increase in the blood reticulocyte count. Normally, as red cell precursors mature, the cells extrude their nucleus at the normal blast stage. The resulting reticulocytes, identified by the supravital stain of their residual ribosomes, persist for about 3 days in the marrow and 1 day in the blood. Erythropoietin shortens the transit time through the marrow, leading to an increase in the number and proportion of blood reticulocytes within a few days.
Figure 1 The architecture of the bone marrow showing the various types of cells.
Figure 2 The pattern for development of various types of blood cells in the bone marrow. (BFU-E—burst-forming unit-erythroid; CFU-GM—colony-forming unit-granulocyte-macrophage; CFU-mega—colony-forming unit-megakaryocyte; EPO—erythropoietin; EPOR—surface component of the erythropoietin receptor; FLT-3L—fms-like tyrosine kinase 3 ligand; G-CSF—granulocyte colony-stimulating factor; GM-CSF—granulocyte-macrophage colony-stimulating factor; IL— interleukin; M-CSF—macrophage colony-stimulating factor; TPO—thrombopoietin; SCF—stem cell factor)
In some conditions, particularly chronic inflammatory diseases, the effectiveness of erythropoietin can be predicted from measurement of the serum erythropoietin level by immunoassay.17,18 It may be cost-effective to measure the level before initiating treatment in patients with anemia attributable to suppressed erythropoietin production, such as patients with HIV infection, cancer, and chronic inflammatory diseases. Several studies have shown that erythropoietin treatment decreases the severity of anemia and improves the quality of life for these patients. In patients with anemia caused by cancer and cancer chemotherapy, current guidelines recommend erythropoietin treatment if the hemoglobin level is less than 10 g/dl.
Thrombopoietin
The development of megakaryocytes from hematopoietic stem cells and the level of platelets in the blood are governed by thrombopoietin.23 Thrombopoietin is produced primarily by the liver and is similar to erythropoietin in structure. However, thrombopoietin has broader biologic effects than erythropoi-etin, stimulating the proliferation and release of hematopoietic stem cells from the bone marrow and prolonging survival of these cells.24 Thrombopoietin signals through its specific receptor, called cMpL, expressed on hematopoietic cells. Plasma thrombopoietin levels are inversely related to the blood platelet count. Deficiencies in thrombopoietin cause thrombocytopenia, and excesses in thrombopoietin cause thrombocytosis. Recom-binant human thrombopoietin is being studied for use in the treatment of thrombocytopenia of diverse causes. Thrombopoi-etin is not yet approved for clinical use.
Granulocyte Colony-Stimulating Factor
G-CSF is a glycosylated protein produced by monocytes, macrophages, fibroblasts, stromal cells, and endothelial cells throughout the body.25 It stimulates the growth and differentiation of neutrophils both in vitro and in vivo. G-CSF levels are normally very low or undetectable but increase with bacterial infections or after administration of bacterial endotoxin.16 G-CSF (the synthesized form is known as filgrastim or lenogras-tim) administration causes a dose-dependent increase in the blood neutrophil count in healthy persons. Studies in animals have shown that G-CSF deficiency causes neutropenia.26 As with erythropoietin, administration of G-CSF leads to an acceleration in the development of neutrophils in the bone marrow, with the neutrophils shifting at an earlier stage than normal from the marrow to the blood.
Table 1 Hematopoietic Growth Factors
|
Factor |
Other Names |
Cell Source |
Chromosome Location |
Function |
|
EPO |
Erythropoietin |
Juxtaglomerular cells |
7q |
Stimulates erythrocyte formation and release from marrow |
|
TPO |
Thrombopoietin; megakaryocyte growth and development factor (MGDF) |
Hepatocytes, renal and endothe-lial cells, fibroblasts |
3q27 |
Stimulates megakaryocyte proliferation and platelet formation |
|
G-CSF |
Granulocyte colony-stimulating factor; filgrastim; lenograstim |
Endothelial cells, monocytes, fibroblasts |
17q11.2-q21 |
Stimulates formation and function of neutrophils |
|
GM-CSF |
Granulocyte-macrophage colony-stimulating factor |
T cells, monocytes, fibroblasts |
5q23-q31 |
Stimulates formation and function of neu-trophils, monocytes, and eosinophils |
|
M-CSF |
Macrophage colony-stimulating factor; colony stimulating factor-1 (CSF-1) |
Endothelial cells, macrophages, fibroblasts |
5q33.1 |
Stimulates monocyte formation and function |
|
IL-1a and IL-1| |
Interleukin-1a and -1|, endogenous pyrogen hemopoietin-1 |
Monocytes, keratinocytes, endothelial cells |
2q13 |
Proliferation of T cells, B cells, and other cells; induces fever and catabolism |
|
IL-2 |
T cell growth factor |
T cells (CD4+, CD8+), large granular lymphocytes (natural killer, or NK, cells) |
4q |
T cell proliferation, antitumor and antimicrobial effects |
|
IL-3 |
Multi-colony stimulating factor; mast cell growth factor |
Activated T cells; large granular lymphocytes (NK cells) |
5q23-q31 |
Proliferation of early hematopoietic cells |
|
IL-4 |
B cell growth factor; T cell growth factor II; mast cell growth factor II |
T cells |
5q23-q31 |
Proliferation of B cells and T cells; enhances cytotoxic activities |
|
IL-5 |
Eosinophil differentiation factor; eo-sinophil colony-stimulating factor |
T cells |
5q23.3-q32 |
Stimulates eosinophil formation; stimulates T cell and B cell functions |
|
IL-6 |
B cell stimulatory factor II; hepatocyte stimulatory factor |
Monocytes, tumor cells, B cells and T cells, fibroblasts, endo-thelial cells |
7p |
Stimulates and inhibits cell growth; promotes B cell differentiation |
|
IL-7 |
Lymphopoietin 1; pre-B cell growth factor |
Lymphoid tissues and cell lines |
8q12-q13 |
Growth factor for B cells and T cells |
|
IL-11 |
Plasmacytoma stimulating factor |
Fibroblasts, trophoblasts, cancer cell lines |
19q13.3-q13.4 |
Stimulates proliferation of early hemato-poietic cells; induces acute-phase protein synthesis |
|
IL-12 |
Natural killer cell stimulating factor |
Macrophages, B cells |
5q31-q33; 3p12-q13.2 |
Stimulates T cell expansion and interferon-gamma; synergistically promotes early hematopoietic cell proliferation |
|
LIF |
Leukemia inhibitory factor |
Monocytes and lymphocytes; stomal cells |
22q |
Stimulates hematopoietic cell differentiation |
|
SCF |
Stem cell factor; kit ligand; steel factor |
Endothelial cells; hepatocytes |
4q11-q20 |
Stimulates proliferation of early hematopoi-etic cells and mast cells |
|
FLT-3 ligand |
fms-like tyrosine kinase 3; STK-1 |
T cells, stromal cells, and fibroblasts |
19q13.3 |
Stimulates early hematopoietic cell differentiation; increases blood dendrite cells |
G-CSF is approved for the treatment of neutropenia after cancer chemotherapy, for acceleration of neutrophil recovery after bone marrow transplantation, for mobilization of hemato-poietic progenitor cells from the marrow to the blood in hema-topoietic transplantation, and for the treatment of severe chronic neutropenia. The usual dosage is 5 mg/kg S.C. daily; higher doses are used to mobilize progenitor cells, and lower doses are used for long-term treatment of neutropenia. A new formulation of G-CSF, in which G-CSF is conjugated to polyethylene glycol to reduce renal clearance, was recently approved for marketing. Its principal advantage is that one injection is sufficient to stimulate marrow recovery after standard doses of cancer chemotherapy. Side effects of either form of G-CSF are principally musculoskeletal pain and headaches during the period of rapid marrow expansion soon after therapy is initiated. Other side effects are uncommon.
Granulocyte-Macrophage Colony-Stimulating Factor
GM-CSF is a glycosylated protein produced by many types of cells, including T cells.28 GM-CSF stimulates formation of neutrophils, monocytes, and eosinophils and may also enhance the growth of early cells of other lineages. In contrast to G-CSF, GM-CSF levels generally do not increase with infections or acute inflammatory conditions,29 and neutropenia does not result from deficiencies of GM-CSF.30 The marrow effects of G-CSF and GM-CSF are similar, but GM-CSF is less potent in elevating the blood neutrophil count.31 GM-CSF (the synthesized form is known as sargramostim or molgramostim) is approved in the United States for acceleration of marrow recovery after bone marrow transplantation or chemotherapy and for mobilization of progenitor cells from the marrow. The usual dosage is 250 mg/m2/day S.C. Its side effects include bone and muscu-loskeletal pain, myalgias, and injection-site reactions.
Figure 3 A model of how hematopoietic growth factors interact with their receptors to initiate cell proliferation. (EPO—erythropoietin; JAK2—Janus kinase 2; SH2—Src homology 2; STAT5—signal transducer and activator of transcription 5)