Classification of Production Defects
Red blood cell production defects cause anemia that is marked by a low absolute reticulocyte count. Examination of the peripheral blood count and the bone marrow aids in classifying these disorders. The marrow characteristically shows one of the following:
1. A normal ratio of myeloid cells to erythroid cells (M: E ratio), normal overall cellularity, and a normal pattern of erythroid maturation.
2. Virtual absence of normal bone marrow elements caused by aplasia (absence of marrow cells) or by replacement of normal marrow elements by fibrosis, solid tumors, granulomas, or leukemia.
3. Erythroid hyperplasia with increased cellularity. Because of defects of erythroid maturation, there is ineffective erythro-poiesis or intramedullary hemolysis. Erythroid precursors die in the marrow, and few cells reach the periphery.
Production Defects Associated with Apparently Normal Bone Marrow
Anemia of Chronic Disease
Definition
The anemia of chronic disease occurs secondary to neoplastic, infectious, and inflammatory diseases and other chronic illnesses, including liver disorders, congestive heart failure, and diabetes mellitus.1,2 Hematocrit values usually range from 27% to 35%, although 20% of patients have hematocrit values below 25%.2
Pathophysiology
The anemia of chronic disease usually results from a combination of slightly shortened red blood cell survival, the sequestration of iron in the reticuloendothelial system, and erythropoi-etin levels that are less than expected for the degree of anemia.1,2 Red blood cells usually have a normal morphologic appearance, although they may occasionally be mildly hypochromic and mi-crocytic. The serum iron and transferrin levels are low, and iron saturation is frequently as low as 15%.1,2 The serum ferritin level is usually normal or elevated.2,3 All these changes can be induced by the inflammatory cytokines (e.g., interleukin-1 [IL-1]; tumor necrosis factor-a; interferons alfa, beta, and gamma; and perhaps transforming growth factor-^).4 Under experimental conditions, these cytokines reduce erythropoietin production, cause hypoferremia, increase serum ferritin levels, impair eryth-ropoiesis, and block release of iron from reticuloendothelial cells.5 Hepcidin, a newly described mediator of iron metabolism, may be the major mediator of the anemia of chronic disease6; hepcidin production is increased up to 100-fold with inflammation. Hepcidin seems to be the long-sought mediator that transmits iron stores to the gut. Hepcidin is secreted when iron stores, primarily in the liver, are increased, and it blocks iron absorption from the gut and causes iron to be trapped in macrophages.6
Diagnosis
Mild anemia, with normal or elevated levels of leukocytes and platelets, in a patient with a chronic illness suggests the diagnosis of anemia of chronic disease. This normocytic or hypochromic and microcytic anemia is easily misdiagnosed as iron deficiency anemia, thalassemia trait, or a sideroblastic anemia. If the diagnosis is uncertain after careful examination of the blood smear, the most useful tests for making the diagnosis are measurement of the serum ferritin level and, in rare cases, bone marrow examination that includes an iron stain [see Table 1 and Figure 1]. In some cases, there is more than one cause of the anemia, and thorough examination of the patient may be required to establish the primary cause. For example, a patient who has anemia of chronic disease resulting from carcinoma of the colon may also be iron deficient because of intestinal bleeding. HIV infection produces complex hematologic effects, including Coombs-positive autoimmune hemolytic anemia, but it also causes anemia of chronic disease in the majority of patients with AIDS.
Table 1 Differential Diagnosis of Hypochromic Anemias
Figure 1 Flowchart shows steps in the diagnosis of anemia caused by production defects. This type of anemia is suggested by a low corrected reticulocyte count or the finding of associated leukocyte or platelet abnormalities on the peripheral blood smear.
Treatment
Identifying and treating the primary disease is the most important part of managing the anemia of chronic disease. Oral or parenteral iron administration is usually not helpful. Erythro-poietin is the standard treatment for patients with anemia of chronic disease. For many patients, administration of pharmaco-logic doses of erythropoietin corrects the anemia of chronic disease by overriding the defect in erythropoietin production. It is useful to obtain a baseline measurement of the plasma erythro-poietin level, because a response to erythropoietin is unlikely in patients whose endogenous levels are above 500 mU/ml. Eryth-ropoietin responses have been reported in patients with rheumatoid arthritis,8 AIDS,9 inflammatory bowel disease,10,11 and cancer.12 To respond optimally, the patient must have adequate available iron stores (i.e., normal or elevated ferritin level or marrow iron stain) [see 5:I Approach to Hematologic Disorders]. Previously, the recommendation was to start the patient on 100 to 150 U/kg subcutaneously three times weekly; however, most physicians give a single subcutaneous dose of 40,000 units of erythropoietin weekly.13
If the hemoglobin level does not rise after 12 weeks, erythro-poietin should be discontinued. A longer-acting form of erythro-poietin, darbepoietin alfa, can be given subcutaneously at doses of 100 ^g weekly or 200 ^g every other week.
Anemia in Severe Renal Disease
Pathophysiology and Etiology
The predominant cause of anemia in renal disease is a deficiency of erythropoietin production by the diseased kidneys. If underlying inflammatory renal disease is present, there may be a component of anemia of chronic disease.14 Anorexia and poor iron intake, frequent blood sampling, and loss of erythrocytes during hemodialysis may produce iron deficiency. Folic acid deficiency, hypersplenism, and secondary hyperparathyroidism with marrow fibrosis4 may also promote anemia.
Anemia in hemodialysis patients can be caused by aluminum toxicity, as well. This anemia was initially identified in patients who had so-called dialysis dementia. Very high plasma aluminum levels probably result from aluminum contamination of the dialysis fluid or gastrointestinal absorption of the aluminum gels taken to bind dietary phosphates. In vitro experiments have shown that aluminum inhibits the growth of the erythroid precursors colony-forming unit-erythroid (CFU-E) and burst-forming unit-erythroid (BFU-E).
Diagnosis
The blood smear should be examined for erythrocyte fragmentation or echinocytosis to exclude other causes of the anemia. The presence of Heinz bodies suggests that oxidative he-molysis has occurred, perhaps caused by oxidants in the he-modialysis fluid.
Treatment
Erythropoietin is the standard treatment for anemic patients with renal disease. Erythropoietin therapy can eliminate the transfusion requirement for patients on hemodialysis and in patients with progressive renal disease who do not yet require he-modialysis. Such treatment significantly improves their quality of life.16 Side effects, such as hyperkalemia and hypertension, occur infrequently. It is customary to start therapy with 50 U/kg of erythropoietin three times weekly, either intravenously or sub-cutaneously, and to increase the dosage as necessary to bring the hemoglobin level to the desired value. Parenteral iron supplementation improves the response. ImFed (a form of iron dex-tran) can be given intramuscularly or intravenously at doses ranging from 100 to 500 mg, with an anticipated frequency of reaction of 4.7%. Ferrlecit (a form of sodium ferric gluconate) can be infused intravenously (125 mg over 1 hour), with the occasional occurrence of hypotension and rash.12 In a study of patients with anemia caused by aluminum toxicity, treatment with I.V. deferoxamine (30 mg/kg I.V. at the end of each dialysis session) produced substantial improvement.17
Anemia Secondary to Other Conditions
Alcohol Abuse
Excessive alcohol ingestion—either acute or chronic—has profound hematologic effects.18 Ingestion of about 80 g of alcohol (one bottle of wine, six pints of beer, or one-third bottle of whiskey) daily may produce macrocytosis,19 stomatocytosis,20 thrombocytopenia,21 vacuolization of proerythroblasts, ringed sideroblasts,20 a sharp drop in serum folic acid levels, and a rise in serum iron levels; it may also impair the reticulocyte response to administered folic acid in a patient known to be folic acid deficient. Acute alcohol ingestion itself does not produce a mega-loblastic anemia.18 It has been postulated that alcohol-induced hematologic toxicity is mediated through acetaldehyde, the major metabolite of ethanol, which is far more toxic and reactive than ethanol. The mechanism for these alcohol-induced abnormalities may be the formation of antibodies against acetalde-hyde-hemoglobin adducts.20 Megaloblasts, macro-ovalocytes, and hypersegmented polymorphonuclear neutrophils (PMNs)usually appear when concomitant folic acid deficiency is present. Chronic alcohol abuse often results in concomitant folic acid or iron deficiency, severe liver disease, GI bleeding, hyper-splenism, and the anemia of chronic disease.
Starvation
Starvation resulting from anorexia nervosa or protein deficiency can cause anemia and even pancytopenia. Hemolysis may also be present [see Figure 2]. The bone marrow biopsy is hypocellular, with a characteristic gelatinous background material consisting of acid mucopolysaccharides. The anemia can occur despite normal folic acid and cobalamin (vitamin B12) levels and can be corrected with proper nutrition.
Hypothyroidism
Hypothyroidism impairs erythrocyte production. The presence of macrocytosis in a hypothyroid patient suggests concomitant dietary folic acid deficiency or pernicious anemia.
Panhypopituitarism
The mild anemia that is associated with severe panhypopitu-itarism can be corrected by replacement of adrenal, thyroid, and gonadal hormones; the enhancing effect of androgens on the action of erythropoietin is well known.
Aging
The hemoglobin levels, red blood cell indices, and leukocyte and platelet counts of healthy older people are similar to those of younger adults; this finding was confirmed in a study of patients who were 84 years of age or older.22 Thus, a workup is required when anemia occurs in such older patients. The evaluation and treatment of anemia in the aged has become increasingly important because the presence of anemia (hemoglobin concentration [Hgb] < 12 g/dl in women and < 13 g/dl in men) is an independent risk factor for decline in quality of life.
Production Defects Associated with Marrow Aplasia or Replacement
The combination of anemia and neutropenia or thrombocy-topenia or the combination of all three of these abnormalities (i.e., pancytopenia) usually indicates that the hematopoietic marrow is damaged. If the marrow cavity is infiltrated but pluripotent stem cells are intact, extramedullary hematopoiesis will often develop in the organs of fetal hematopoiesis (i.e., spleen, liver, and distal bones).
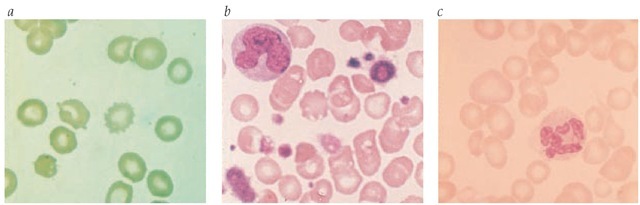
Figure 2 The peripheral smear changes seen in severe liver disease or starvation (a) include distinct variation in size and shape of red blood cells; both sharply spiculed cells (spur cells) and scalloped erythrocytes are prominent. The leukoerythroblastic blood smear (b) indicates marrow replacement with extramedullary hematopoiesis. It is characterized by variation in the size and shape of red blood cells, by the presence of nucleated red blood cells in the peripheral blood, by giant platelets, and by immaturity in the myeloid series. In folic acid or cobalamin deficiency (c), the smear is characterized by variation in erythrocyte size and by distinct macrocytosis. Occasionally, fish-tailed erythrocytes are present, along with hypersegmented neutrophils.
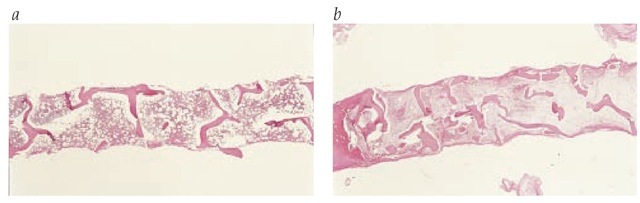
Figure 3 Shown are (a) biopsy of normal bone marrow and (b) biopsy of bone marrow from a patient with aplastic anemia showing almost complete aplasia.
Pancytopenia can be congenital or acquired. The finding of combined cytopenias or of immature cells in the blood (myelo-cytes, metamyelocytes, and erythroblasts)—that is, a leukoeryth-roblastic blood smear—suggests extramedullary hematopoie-sis [see Figure 2]. These findings are an indication for bone marrow aspiration and biopsy.