Geoscience Reference
In-Depth Information
Loss of energy from the solar spectrum at particular wavelengths occurs
because of
molecular absorption
. Because of their structure, molecules absorb
radiation at particular wavelengths, and for some molecules in the atmosphere
these wavelengths are within the solar spectrum. For example, an ozone mol-
ecule (O
3
) has three oxygen atoms arranged in an isosceles triangle in the
two-dimensional plane. Only two sides of the ozone triangle are occupied by
chemical bonds, so two of the atoms are not bound to each other. With this
structure, the ozone molecule has three vibrational degrees of freedom (sym-
metric stretching, bending, and asymmetric stretching) and three rotational de-
grees of freedom (one each around the
x-
,
y- ,
and
z
-axes). As a result, ozone
absorbs radiation at certain wavelengths—actually, in certain wavelength
bands, since temperature and precipitation variations broaden the absorption
bands—that excite these allowed modes of motion, removing the energy from
the radiation spectrum at those certain wavelengths and converting it into the
kinetic energy of vibration or rotation.
Ozone absorption is strong in the ultraviolet part of the spectrum. Note
in
Figure 4.4
that the solar radiation incident at the top of the atmosphere is
depleted in wavelengths shorter than about 0.3 mm by the time it reaches the
ground. There is also significant absorption at visible wavelengths by ozone,
in the Chappuis bands from 0.44 to 0.74 mm. Atmospheric water vapor (H
2
O)
is responsible for the broad absorption bands centered near 0.9 mm, 1.1 mm,
1.4 mm, and 1.9 mm. Molecular oxygen (O
2
) and carbon dioxide (CO
2
) also
contribute to shortwave absorption.
Table 4.1
lists many of the principal ab-
sorbing molecules in the earth's atmosphere and the wavelengths at which they
absorb radiation.
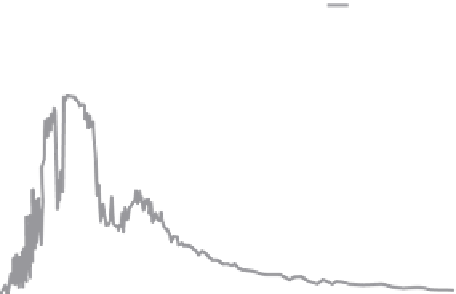
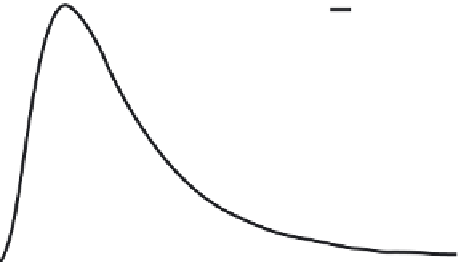
Observed spectra of longwave emission from the earth are shown in
Figure
4.5.
The black curve is the observed emission at the surface, before the energy
passes through the atmosphere. The jagged gray line is earth's spectral irradi-
ance recorded by a satellite outside the atmosphere. This radiation is known as
the
OLR
, or
outgoing longwave radiation
.
30
Top of the
atmosphere
25
Surface
20
15
10
5
0
0
10
20
30
40
50
Wavelength (µm)
Figure 4.5 Terrestrial emission of radiation measured at the surface
(black line) and at the top of the atmosphere (gray line). Adapted
from Kiehl and Trenberth (1997).