Geoscience Reference
In-Depth Information
C EVAPORATION
of the water particles. This energy is often acquired by
the removal of heat from the immediate surroundings,
causing an apparent heat loss (
latent heat
), as discussed
on p. 55, and a consequent drop in temperature. The
latent heat of vaporization needed to evaporate 1 kg of
water at 0°C is 2.5
10
6
J. Conversely, condensation
releases this heat, and the temperature of an airmass in
which condensation is occurring is increased as the
water vapour reverts to the liquid state.
The diurnal range of temperature can be moderated
by humid air, when evaporation takes place during the
day and condensation at night. The relationship of
saturation vapour pressure to temperature (Figure 2.14)
means that evaporation processes limit low latitude
ocean surface temperature (i.e. where evaporation is at
a maximum) to values of about 30°C. This plays an
important role in regulating the temperature of ocean
surfaces and overlying air in the tropics.
The rate of evaporation depends on a number of
factors, the two most important of which are the differ-
ence between the saturation vapour pressure at the water
surface and the vapour pressure of the air, and the
existence of a continual supply of energy to the surface.
Wind velocity also affects the evaporation rate, because
Evaporation (including transpiration from plants) pro-
vides the moisture input into the atmosphere; the oceans
provide 87 per cent and the continents 13 per cent.
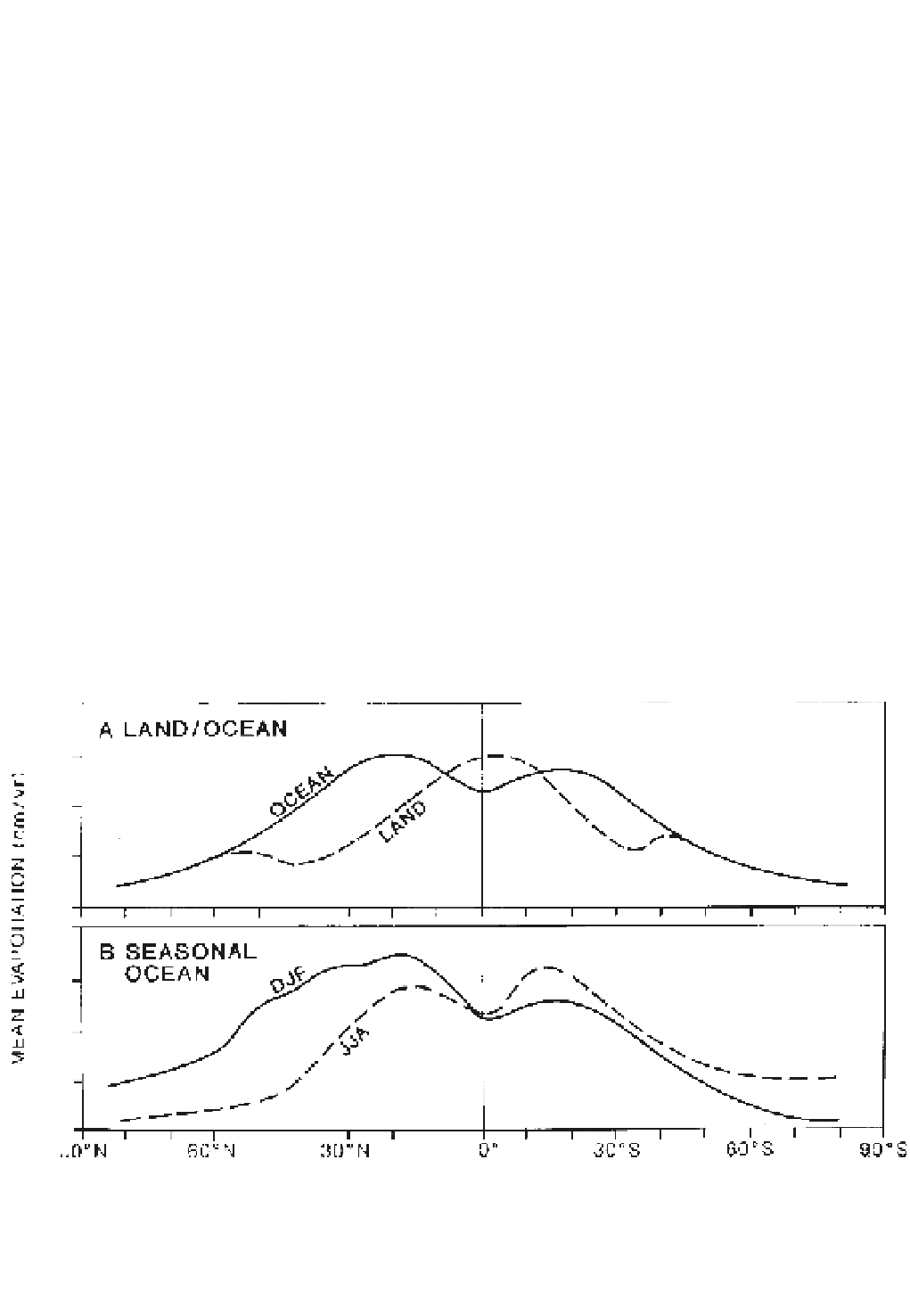
The highest annual values (1500 mm), averaged
zonally around the globe, occur over the tropical oceans,
associated with trade wind belts, and over equatorial
land areas in response to high solar radiation receipts
and luxuriant vegetation growth (Figure 4.5A). The
larger oceanic evaporative losses in winter, for each
hemisphere (Figure 4.5B), represent the effect of out-
flows of cold continental air over warm ocean currents
in the western North Pacific and North Atlantic (Figure
4.6) and stronger trade winds in the cold season of the
southern hemisphere.
Evaporation requires an energy source at a surface
that is supplied with moisture; the vapour pressure in
the air must be below the saturated value (
e
s
); and air
motion removes the moisture transferred into the surface
layer of air. As illustrated in Figure 2.14, the saturation
vapour pressure increases with temperature. The change
in state from liquid to vapour requires energy to be
expended in overcoming the intermolecular attractions
2000
A LAND/OCEAN
1500
1000
500
0
2000
B SEASONAL
OCEAN
1500
1000
500
0
90˚N
60˚N
30˚N
0˚
30˚S
60˚S
90˚S
Figure 4.5
Zonal distribution of mean evaporation (mm/year): (A) annually for the ocean and land surfaces, and (B) over the oceans
for December to February and June to August.
Sources
: After Peixoto and Oort (1983). From
Variations in the Global Water Budget
, ed. A. Street-Perrott, M. Beran and R. Ratcliffe (1983),
Fig. 22. Copyright © D. Reidel, Dordrecht, by kind permission of Kluwer Academic Publishers. Also partly from Sellers (1965).