Geoscience Reference
In-Depth Information
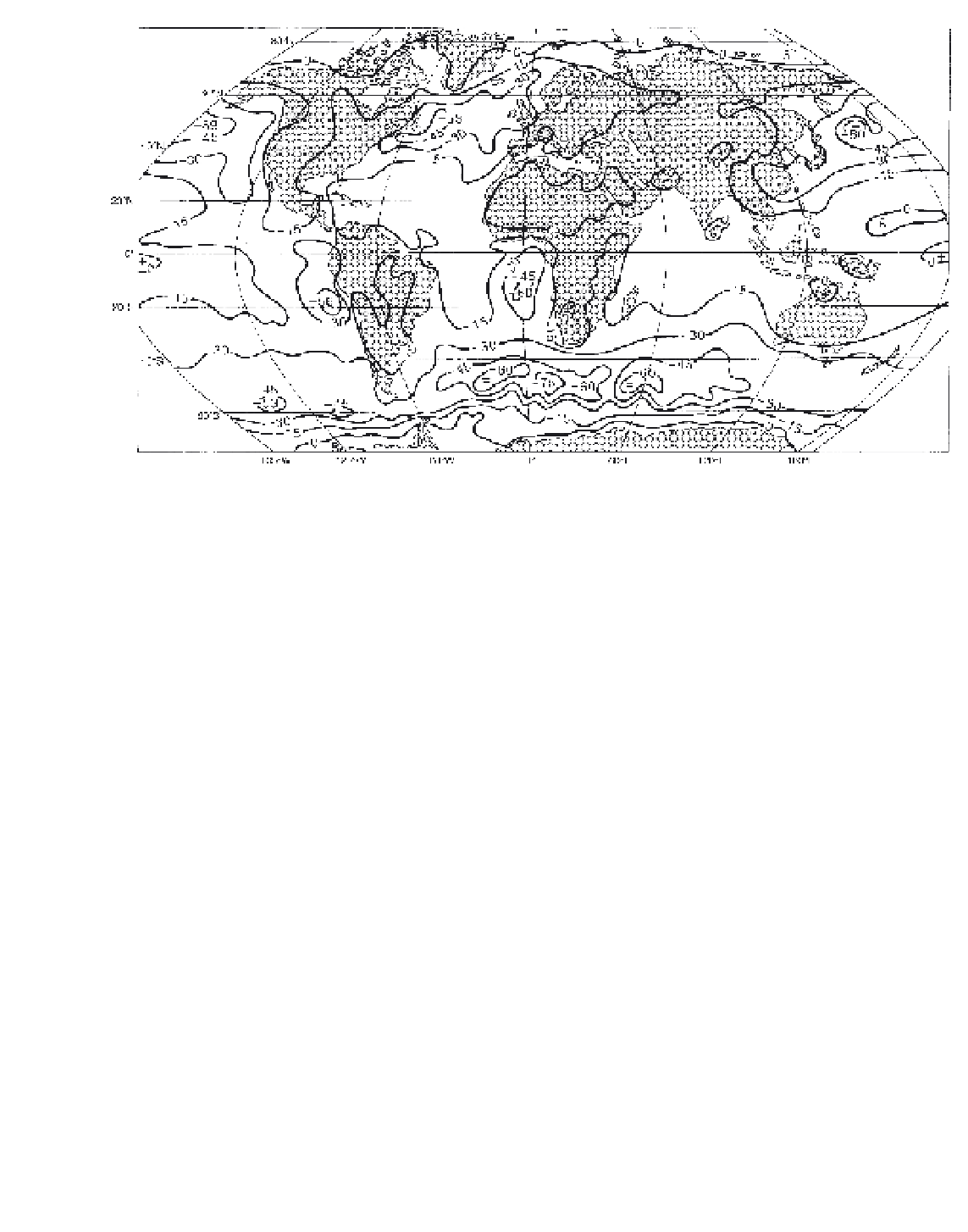
Figure 5.12
Mean annual net cloud forcing (W m
-2
) observed by the
Nimbus-7
ERB satellite for the period June 1979 to May 1980.
Source
: Kyle
et al
. (1993) From
Bulletin of the American Meteorological Society
, by permission of the American Meteorological Society.
might grow at the expense of small ones. However,
observations show that the distribution of droplet size in
a cloud tends to maintain a regular pattern; the average
radius is between 10 and 15 µm, and few are larger
than 40 µm. A further idea was that atmospheric tur-
bulence might bring warm and cold cloud droplets
into close conjunction. The supersaturation of the
air with reference to the cold droplets and the under-
saturation with reference to the warm ones would cause
the latter to evaporate and cold droplets to develop at
their expense. However, except perhaps in some tropical
clouds, the temperature of cloud droplets is too low
for this differential mechanism to operate. Figure 2.14
shows that, below about -10°C, the slope of the satu-
ration vapour pressure curve is low. Another theory
was that raindrops grow around exceptionally large
condensation nuclei (observed in some tropical storms).
Large nuclei do experience a more rapid rate of initial
condensation, but after this stage they are subject to the
same limiting rates of growth that apply to all cloud
drops.
Current theories for the rapid growth of raindrops
involve either the growth of ice crystals at the expense
of water drops, or the coalescence of small droplets by
the sweeping action of falling drops.
1 Bergeron-Findeisen theory
This widely accepted theory is based on the fact that at
subzero temperatures the atmospheric vapour pressure
decreases more rapidly over an ice surface than over
water (Figure 2.14). The saturation vapour pressure
over water becomes greater than over ice, especially
between temperatures of -5 and -25°C, where the dif-
ference exceeds 0.2 mb. If ice crystals and supercooled
water droplets exist together in a cloud, the drops tend
to evaporate and direct deposition takes place from the
vapour on to the ice crystals.
Freezing nuclei
are necessary before ice particles can
form - usually at temperatures of about -15 to -25°C.
Small water droplets can, in fact, be supercooled in pure
air to -40°C before spontaneous freezing occurs. But
ice crystals generally predominate in clouds where
temperatures are below about -22°C. Freezing nuclei
are far less numerous than condensation nuclei; there
may be as few as 10 per litre at -30°C and probably
rarely more than 1000. However, some become active
at higher temperatures. Kaolinite, a common clay
mineral, initially becomes active at -9°C and on sub-
sequent occasions at -4°C. The origin of freezing nuclei
has been a subject of much debate but it is generally
considered that very fine soil particles are a major