Geoscience Reference
In-Depth Information
whereas in New York City the observed decrease
began in the same year (1964) -
prior
to the air
pollution control regulations there. Daily mean
concentrations in most of Western Europe and
North America now seldom exceed about
0.04ppm (125
NITROGEN
DIOXIDE
(NO
2
)
ULTRAVIOLET
LIGHT
+
OXYGEN
ATOM
(O)
g m
-3
), but where coal is still
widely used for domestic heating and industry
and there is heavy diesel traffic, as in Eastern
Europe, Asia and South America, levels may be
5-10 times higher.
Urban complexes in many parts of the world
are significantly affected by pollution resulting
from the combustion of gasoline and diesel fuel by
vehicles and aircraft, as well as from petrochemical
industries. Los Angeles, lying in a topographically
constricted basin and often subject to temperature
inversions, is the prime example of such pollution,
although this affects all modern cities. Even with
controls, 7 percent of the gasoline from private
cars is emitted in an unburned or poorly oxidized
form, another 3.5 percent as photochemical smog
and 33-40 percent as carbon monoxide. Smog
involves at least four main components: carbon
soot, particulate organic matter (POM), sulfate
(SO
4
2-
) and peracyl nitrates (PANs). Half of the
aerosol mass is typically POM and sulfate. How-
ever, there are important regional differences.
For example, the sulfur content of fuels used in
California and Australia is lower than in the
eastern United States and Europe, and NO
2
emissions greatly exceed those of SO
2
in
California. The production of the Los Angeles
smog, which, unlike traditional city smogs, occurs
characteristically during the daytime in summer
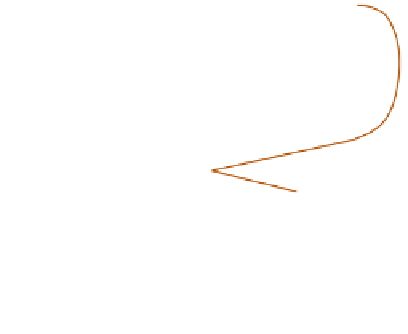
and autumn, is the result of a very complex chain
of chemical reactions termed the disrupted
photolytic cycle (
Figure 12.22
). Ultraviolet
radiation dissociates natural NO
2
into NO and O.
Monatomic oxygen (O) may then combine with
natural oxygen (O
2
) to produce ozone (O
3
). The
ozone in turn reacts with the artificial NO
to produce NO
2
(which goes back into the
photochemical cycle forming a dangerous positive
feedback loop) and oxygen. The hydrocarbons
produced by the combustion of petrol combine
μ
NITRIC
OXIDE
(NO)
+
OXYGEN
(O
2
)
OXYGEN
(O
2
)
+
OZONE
(O
3
)
SMOG
HYDRO-
CARBON
FREE
RADICAL
(H
c
O
*
)
HYDROCARBONS
(H
c
)
Figure 12.22
The NO
2
photolytic cycle disrupted
by hydrocarbons to produce photochemical smog.
Sources: US DHEW (1970) and Oke (1978).
with oxygen atoms to produce the hydrocarbon
free radical HcO*, and these react with the
products of the O
3
-NO reaction to generate
oxygen and photochemical smog. This smog
exhibits well-developed annual and diurnal cycles
in the Los Angeles basin (see
Figures 12.19C
and
D
). Annual levels of photochemical smog
pollution in Los Angeles (from averages of the
daily highest hourly figures) are greatest in late
summer and autumn, when clear skies, light
winds and temperature inversions combine with
high amounts of solar radiation. The diurnal
variations in individual components of the
disrupted photolytic cycle indicate complex
reactions. For example, an early morning
concentration of NO
2
occurs due to the buildup
of traffic and there is a peak of O
3
when incoming
radiation receipts are high. The effect of smog is
not only to modify the radiation budget of cities
but also to produce a human health hazard.
Evolving state and city regulations in the
United States have given rise to considerable
differences in the type and intensity of urban
pollution. For example, Denver, Colorado,
situated in a basin at 1500m altitude, regularly
had a winter 'brown cloud' of smog and high
summer ozone levels in the 1970-1980s. By
the beginning of this century, substantial