Geoscience Reference
In-Depth Information
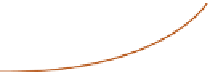
Animals
Plants
Residues
Symbiotic
organisms
Mineralization
Organic P
(in humus)
Soluble P
Immobilization
Inorganic P
compounds
Adsorbed P
Marine birds
Animals
Phosphate
rocks
Plants
Guano
Bones
Erosion
Excreta
Bacteria
Phosphates
Fish
Protein
synthesis
Shallow marine
sediments
Losses
to deep sea
sediments
oceans (80
10
12
kg), rocks such as apatite (20
10
12
recycling of this nutrient. Phosphorus availability depends
mainly on the pH of the soil. Under acid soil conditions,
phosphorus is quickly precipitated as iron and alumin-
ium phosphates; both of these cations are more soluble
at low pH. Under alkaline soil conditions, phosphorus
is precipitated as calcium phosphates in the presence of
the large calcium concentrations at high pHs. In iron,
aluminium, and calcium phosphates, phosphorus is held
in a chemical form which is not available to plants. It is
kg) and biomass (0.1
10
12
kg).
Phosphorus is released from rock minerals by chemical
and microbiological weathering. However, the major part
of the phosphorus in soils is in the organic matter, largely
as inositol phosphates, and the PO
4
3-
will be released as
decomposition and mineralization processes take place.
The fate of PO
4
3-
released by both mineral weathering and
organic decomposition is crucial for the uptake and