Geoscience Reference
In-Depth Information
is the same as the atomic weight; for divalent cations it
is half the atomic weight (Ca
2+
,Mg
2+
) and for trivalents
one-third (Fe
3+
,Al
3+
). Since the amounts involved are
very small, the term
milliequivalent
(EW/1,000) is used.
The unit me 100 g
-1
therefore represents the number of
milligrams of particular elements which can be held
by 100 g of a particular soil. In recent years, however, a
new notation has come into prominence for quantifying
CEC. This is
cmol
c
kg
-1
. The numerical values of me
100g
-1
and cmol
c
kg
-1
are identical. The average electric
charges (CEC) on the common clay minerals are given in
The net negative charge on the clay colloids is balanced
by
exchangeable cations
which are attracted to the surface
of the clay particles. These are positively charged ions in
the soil solution (H
+
,Ca
2+
,Mg
2+
,K
+
,Na
+
). They are
termed 'exchangeable' because one cation can be readily
replaced by another of equal valence, or by two of half
the valence of the original one. For example, if a clay
containing sodium as the exchangeable cation is washed
with a solution of calcium chloride, each calcium ion will
replace two sodium ions, and the sodium will be washed
out in solution. This process is called
cation exchange
or
base exchange
. It can be written as the chemical equation:
with the acidic cations, aluminium and hydrogen. The
influence of hydrogen ions on the exchange sites was
originally thought to give soils acidic properties, but it was
later found that acid clays had aluminium rather than
hydrogen as the exchangeable ion. In very acid soils the
clay minerals themselves start to dissociate, releasing
aluminium which can then move on to the soil complex.
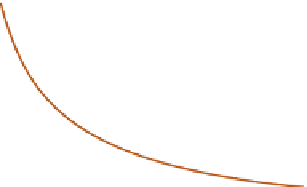
The process of cations fixing themselves on to exchange
sites on colloids is termed
adsorption
. The cations are not
all held in a layer right at the clay surface but are present
inner layer is the highest concentration of cations at the
colloid surface, attracted by coulomb electrical forces,
and is the
Stern layer
; the outer layer is a diffuse 'cloud'
of cations whose thermal energy makes them diffuse away
from the colloid surface.
Table 19.7
gives the cation exchange data for five
contrasting soils. The values for the four commonest base
cations (Ca, Mg, K, Na) are given, together with those for
hydrogen (H). The total cation exchange capacity is the
sum of these five ions, and the percentage base saturation
(per cent BS) is the proportion of the CEC occupied by
these four base cations. The pH values are directly related
to per cent BS.
Na
2
Clay + CaCl
2
= Ca Clay + 2NaCl
COLLOIDAL PROPERTIES OF
HUMIC COLLOIDS
The total quantity of exchangeable cations held is the
cation exchange capacity
(CEC). The predominant ex-
changeable cations in soils are calcium and magnesium,
with lesser amounts of potassium and sodium. Alu-
minium and hydrogen are common in acid soils. The
proportions of these cations found on the colloids of
any particular soil are governed by the parent rock and
by the nature and intensity of weathering and leaching.
Calcareous soils over limestone will contain mostly
calcium. Clays deposited in sea water will have mostly
magnesium and sodium. Leaching removes the cations
which form bases (e.g. calcium, sodium), leaving a clay
The values of the cation exchange capacities for
clay minerals range from a low of about 5 me 100 g
-1
Distance from
colloid surface
0
Charge
(me 100g
-1
)
Clay mineral
(cmol
c
kg
-1
)
Source of charge
Kaolinite
5-15
Broken bands
Ionization of OH
Illite, chlorite
20-40
Ion substitution
Montmorillonite
80-100
Ion substitution
the outer diffuse layer.
Vermiculite
100-150
Ion substitution