Geoscience Reference
In-Depth Information
transformation and modification. We have seen already
that energy can exist in a number of forms, and as a
general principle energy will be transferred from areas
of high energy status to areas of lower energy status
in an attempt to eradicate the differences. Thus energy
differences expressed by the level of temperature in two
bodies, such as the air and the soil, tend to be reduced over
time as heat is transferred from the hotter to the cooler
body. In this way the soil is heated during the day when
the air is warm and loses heat energy back to the air at
night when the atmosphere is cool
(
Figure 2.4
)
.
In the case of thermal energy, three main methods of
transfer can be identified: radiation, convection and
conduction.
Radiation
is the process by which energy is
transmitted through space, mainly by the mechanism of
electromagnetic waves.
Convection
involves the physical
movement of substances containing heat, such as water
or air, and is not possible in a solid.
Conduction
is the
transfer of heat through a medium from molecule to
molecule (see box below).
These three processes of transfer are often closely
related. Thus energy may be conducted through the soil
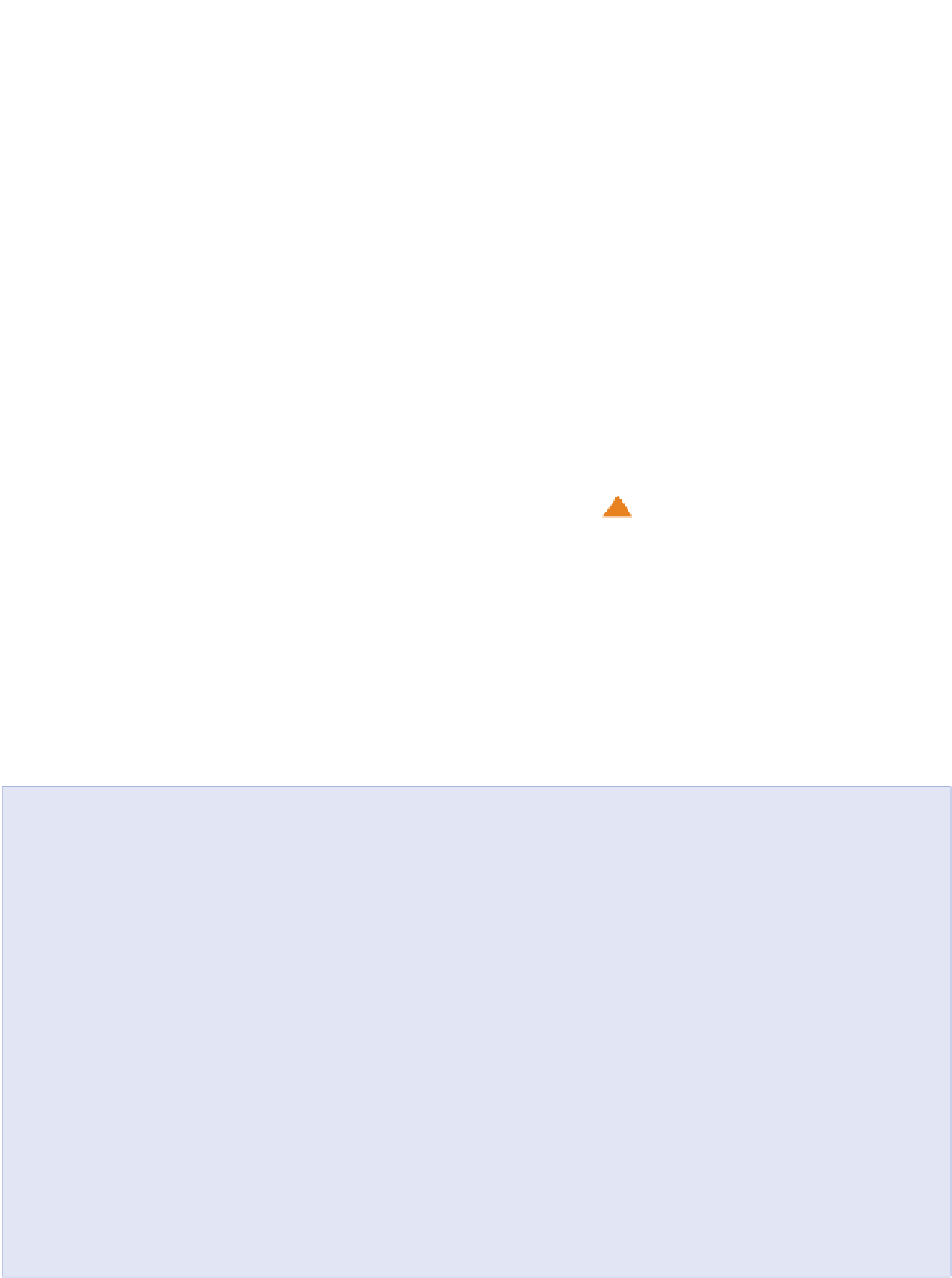
Day
Night
Surface
radiant
energy
Radiant
energy
Surface
radiant
energy
Counter
radi
a
tion
Latent
La
te
nt
Sensible
Sen
si
ble
Air
Soil
Warmer
Cooler
Cooler
Warmer
Heat energy
Heat energy
Figure 2.4
Energy exchange at the soil surface. The size of the arrows is only approximately to scale. Sensible and latent heat
flows at night can be upwards if the temperature and humidity gradients are the same as in daytime.
Heat and temperature
KEY CONCEPTS
We may think that heat and temperature are the same thing. Something that has a lot of heat may be expected to
have a high temperature, but it is not as simple as that. Heat is a measure of the internal energy of a body or substance.
It is due to the velocity of vibration of the molecules of which that body is composed. If a metal rod is heated at one
end, it will gain heat energy and the molecular movement will be faster. These molecules will collide with the cooler
and more slowly moving neighbouring molecules of the unheated part of the rod, passing on some of their energy.
This process continues along the rod even though the molecules themselves do not change position. This is the
process of conduction of heat.
Temperature is the measure of the average speed or kinetic energy of the molecules, with higher temperatures being
associated with greater speed. In the upper atmosphere, gas molecules move at high speeds and so have high
temperatures. As the density of air is low at these heights and there are few molecules the heat energy is small.
We use a variety of scales to measure temperature. The most basic one is the Kelvin scale. For its base it uses the
temperature at which atoms and molecules possess a minimum amount of energy and theoretically no thermal motion.
This is termed absolute zero or 0K. On this scale the freezing point of water is 273K. More popularly we have the
Celsius or Centigrade scale based on 0
s
C as the freezing point of water and 100
s
C as its boiling point. The Fahrenheit
scale uses 32
s
and 212
s
for these boundaries.