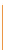Geoscience Reference
In-Depth Information
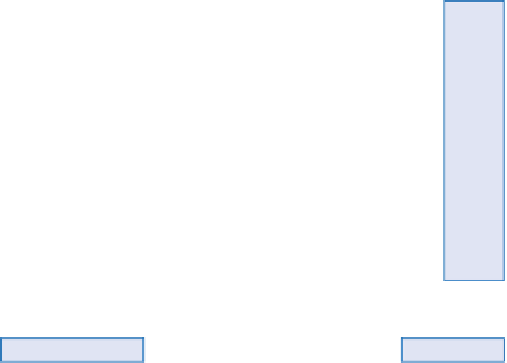
OLIVINE
ANORTHITE
Ca—feldspar
PYROXENE
Augite
Ca—Na feldspar
AMPHIBOLE
Horrnblende
BIOTITE
ALBITE
Na—feldspar
ORTHOCLASE
K—feldspar
MUSCOVITE
QUARTZ
sediments in the San Gabriel mountains, California. They are
thought to form through mechanical action associated with
de/rehydration cycles, salt weathering and wind in the semi-
arid and coastal environment.
Photo: Ken Addison
closely linked with magma geochemistry and melt
temperatures.
Most minerals dissolve slowly in water, but those
dominated by ionic bonds, e.g. mafic minerals Mg
++
,Fe
++
,
CaAl
2
Si
2
O
8
(calc-plagioclase), K
+
(potassium), Na
+
(sodium) and Ca
+
(calcium), are more susceptible to
solution
than felsic minerals dominated by covalent
bonds, e.g. KAlSiO
3
O
8
(orthoclase), KAl
2
(OH)
2
Si
3
AlO
10
(muscovite) and (SiO
2
) quartz. Al
2
SO
3
(alumina) is
solvent
. Hydrogen ion concentration is expressed as the
pH
of water, which is neutral when pH = 7·0,
acid
at less
than 7·0 and
alkaline
at over 7·0. Chemical weathering
potential increases inversely with pH and proportionally
with increasing equilibrium solubility of minerals and
temperature, until saturation is reached.
Ti
Fe
Al
Si
Anatase
Goethite
Haematite
Gibbsite
Boehmite
Amorphous
OLIVINE
AUGITE
HORNBLENDE
^
Hydrous
Oxides
^
VOLCANIC
GLASS (basic)
ZEOLITE
Amorphous Fe
Hydrous Al
Oxides Si
Gibbsite
Allophane
H
+
,Ca
2+
K
+
Ca
2+
Ca
2+
Illite (3-octohedral)
BIOTITE
-SiO
2
Montmorill-
onite
Vermiculite Clay
+SiO
2
MUSCOVITE
H
+
,Ca
2+
Illite (2-octohedral)
K
+
VOLCANIC
GLASS (acid)
FELDSPARS
Amorphous Al
Hydrous
Oxides Si
Gibbsite
Figure 13.9
Weathering sequence and
rock-forming minerals.
Source: After Selby (1993)
QUARTZ
Silicic acid
Chalcedonite
Silicic acid
Secondary
Quartz