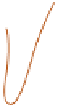Geoscience Reference
In-Depth Information
tion, associated with high water volumes circulating at
mid-ocean ridges, with water : rock ratios of 10-100 : 1.
Metasomatism takes large volumes of basaltic and other
minerals into solution, infusing new species into the crust,
depositing others around and beyond the vents and taking
yet others out of solution. Three associations are found,
reflecting the peridotite qgabbro qbasalt layering
towards the ocean floor and parallel temperature/pressure
decline.
Serpentin
e - Mg
3
Si
2
O
5
(OH)
4
- forms on the
hydration of olivine in peridotite above 400°C.
Hornblende
- (Na,Ca)
2
(Mg,Fe,Al)
5
O
22
(OH)
2
- forms in gabbro at
200-400
Hydrothermal circulation and
metasomatism
Ocean water comes into contact with the sea bed but also
circulates to depths of several kilometres in oceanic crust,
penetrating the basalt and gabbro layers and maybe also
reaching upper-asthenosphere peridotite. It gains access
to the lithosphere via faults and fractures generated by
mid-ocean ridge rifting, post-formational cooling con-
traction and subsidence. This process occurs over a very
wide area, perhaps over 30 per cent of the ocean floor,
driven by mantle convection. Typical heat fluxes exceed
50 mW m
-2
in 'new' crust up to 50 Ma old and 200-250
mW m
-2
at the ridges. This draws water in over a wide area
and pumps it in concentrated
hydrothermal plumes
through axial vents. Although the process is known simply
as
hydrothermal circulation
, it is clear that water-rock-
magma reactions occur in a number of different
environments and styles, determined by the thermal
environment. Even without mantle convection,
cold sea-
water weathering
occurs at the sea bed. As expected, this
occurs mostly through hydration, which produces
hydrated aluminosilicate clay minerals, but oxidation also
occurs, forming oxide films on Fe and Mn minerals
relevant to
red clays
, described below.
At temperatures above 200
C, and a whole cluster of new minerals form
at similar temperatures in basalt, including
albite
-
NaAlSi
3
O
8
-
chlorite
- (Mg,Fe,Al)
3
(Si,Al)
2
O
5
(OH) - and
are key minerals of the metasomatic facies
serpentinite
,
amphibolite
and
greenschists
.
These reactions portray
par excellence
the vital
contribution of hydration (OH), the magmatic minerals
Fe, Mg, Al, Si, Ca and Na, and solid solutions to ocean and
oceanic crust geochemistry. Sea water becomes enriched
by chloride and the soluble minerals Mn and Fe but
depleted of Mg. Hydrogen sulphide (H
2
S), barium
sulphate (BaSO
4
), anhydrite (CaSO
4
) and insoluble metal
sulphides of Cu, Fe and Zn are precipitated - particularly
by
black smokers
or hot plumes of suspended and dissolved
minerals - to form important mineral ore deposits. Vents
also stimulate their own chemosynthesizing ecosystems
and hydrothermal circulation exhales magmatic gases,
including hydrogen (H), helium (He), methane (CH
4
) and
carbon monoxide (CO) (
Figure 12.17
).
C hydration may form the
lowest-grade metamorphic facies,
zeolite
. Precipitation of
solutes also occurs here by
reverse weathering
, involving
hydrothermal minerals themselves together with dissolved
minerals sourced elsewhere. High temperature/low water
volume reactions drive metamorphism, particularly in
subduction zones. This is quite distinct from mineraliza-
HHe
CH
4
CO
Km
0
OCEAN
WATER
Hydrothermal plume
1
H
2
S
Black smoker
Cu Fe Zn
CaSO
4
BaSO
4
Fe
Cold water weathering
Mn
2
3
BASALT
4
Figure 12.17
Hydrothermal circulation
around a mid-ocean ridge,
indicated by solid arrows.
as the cracking plane, above
which layered ocean crust is
stretched and cracked.
Magma
5
GABBRO
6
Cracking plane
7