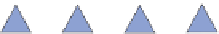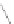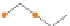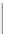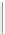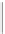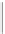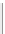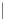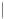Geoscience Reference
In-Depth Information
SILICATE STRUCTURE
AND FORMULA
MINERAL EXAMPLES, CRYSTAL AND
CLEAVAGE CHARACTER, SPECIFIC GRAVITY
SILICATE TETRAHEDRON
NONE
Olivine, garnet, zircon. Dense, equidimensional
crystals
simplified
as
SiO
4
Specific gravity = 3.5-4.0
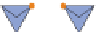
RING SILICATES
2
Beryl, tourmaline. Columnar (prismatic) crystals;
cleavage between rings and across columns.
Si
6
O
18
2.7-3.2
CHAIN SILICATES
(a) Single chain
2
Pyroxenes. Dense, equidimensional crystals;
cation bonding inhibits cleavage.
SiO
3
3.0-4.0
(b) Double chain
2 - 3
Amphiboles. Well-developed cleavage, aided by
weak cation bonds
between
chains.
Si
4
O
11
2.7-3.6
Micas, clay minerals, talc, serpentine.
Low density, excellent cleavage
between
sheets.
3
SHEET SILICATES
2.6-3.3
Si
2
O
5
FRAMEWORK SILICATES
4
Quartz, feldspars, zeolite. Less dense but strong,
three-dimensional bonding. Cleavage absent in
quartz, present in feldspars.
Complex, 3-dimensional
structures
SiO
2
Figure 12.2
The structure and characteristics of silicates and some representative silicate minerals. The silicate tetra-hedron
of four large oxygen anions and a single small silicon cation and its simplified form are shown in the first panel. Subsequent
structures are shown with the number of shared oxygen anions and their silicon-oxygen formulae.