Geoscience Reference
In-Depth Information
Industrial
processes
16.8%
Power stations
21.3%
Transport fuels
14.0%
Waste disposal
and treatment
3.4%
10.0%
12.5%
Agricultural
byproducts
Land use and
biomass burning
10.3%
11.3%
Residential, commercial
and other sources
Fossil fuel retrieval,
processing, and
distribution
62.0%
40.0%
29.5%
20.6%
1.1%
4.8%
6.6%
1.5%
8.4%
2.3%
29.6%
5.9%
19.2%
9.1%
18.1%
12.9%
26.0%
Carbon Dioxide
(72% of total)
Methane
(18% of total)
Nitrous Oxide
(9% of total)
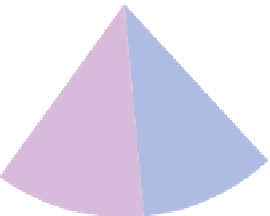
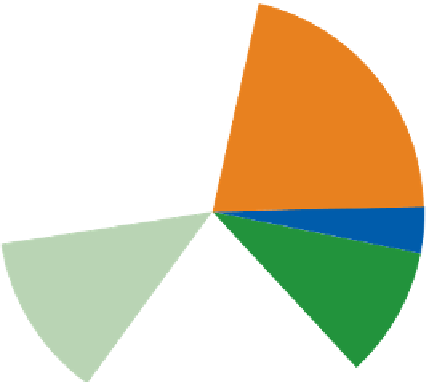
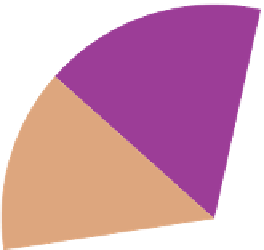
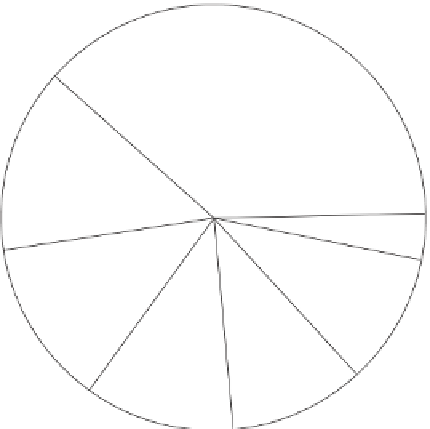
Figure 9.15
Annual greenhouse gas emissions, by sector. The lower diagram shows the sources of each of the three main
greenhouse gases.
Source: Wikipedia, 2007
Conversely the emission of sulphur by-products from
biomass and fossil fuel burning into the atmosphere
leads to the formation of sulphate aerosols which have a
strong regional effect on climate. The suspended particles
increase scattering and reflection of insolation at a greater
rate than they absorb outgoing long-wave radiation, so
leading to cooling. Incorporation of sulphate aerosols
into climate models does improve the temperature
predictions of the models in comparison with observed
temperature changes of the instrumental period. The
preliminary fourth Assessment Report of the Inter-
Governmental Panel on Climate Change states that 'It is
likely
that increases in greenhouse gas concentrations
alone would have caused more warming than observed
because volcanic and anthropogenic aerosols have offset
some warming that would otherwise have taken place.'
The enhanced greenhouse effect may be modified to some
extent but the sulphate aerosols will sustain the acidity of
precipitation.
The appearance of CFCs in the atmosphere has had
two disturbing impacts which were never foreseen. CFCs
are extremely stable molecules which gradually disperse
throughout the atmosphere. They were made artificially
because of their suitability for use in foam packaging,
aerosol propellants, solvents and refrigerants. They are
destroyed by the action of ultra-violet light in the
stratosphere, yielding free chlorine atoms. The highly
reactive chlorine reacts with ozone to produce chlorine
monoxide and oxygen. Chlorine monoxide is unstable,
reacting with free oxygen atoms to form a further oxygen