Geoscience Reference
In-Depth Information
Acid rain is a complex problem which is likely to remain as long as the atmosphere is polluted, though the decrease
in sulphur emissions has led to some improvement in acidity. In a UK survey (2005) half the twenty-two sites being
monitored were showing signs of recovery. Nevertheless, it is an international problem; the areas affected are not
necessarily the source of the pollution. As with the enhanced greenhouse effect, we are still not certain in detail
about the processes at work and hence prediction is difficult. Ironically the sulphates formed in the rainfall acidification
process can reflect insolation and so their decline may help to enhance greenhouse warming.
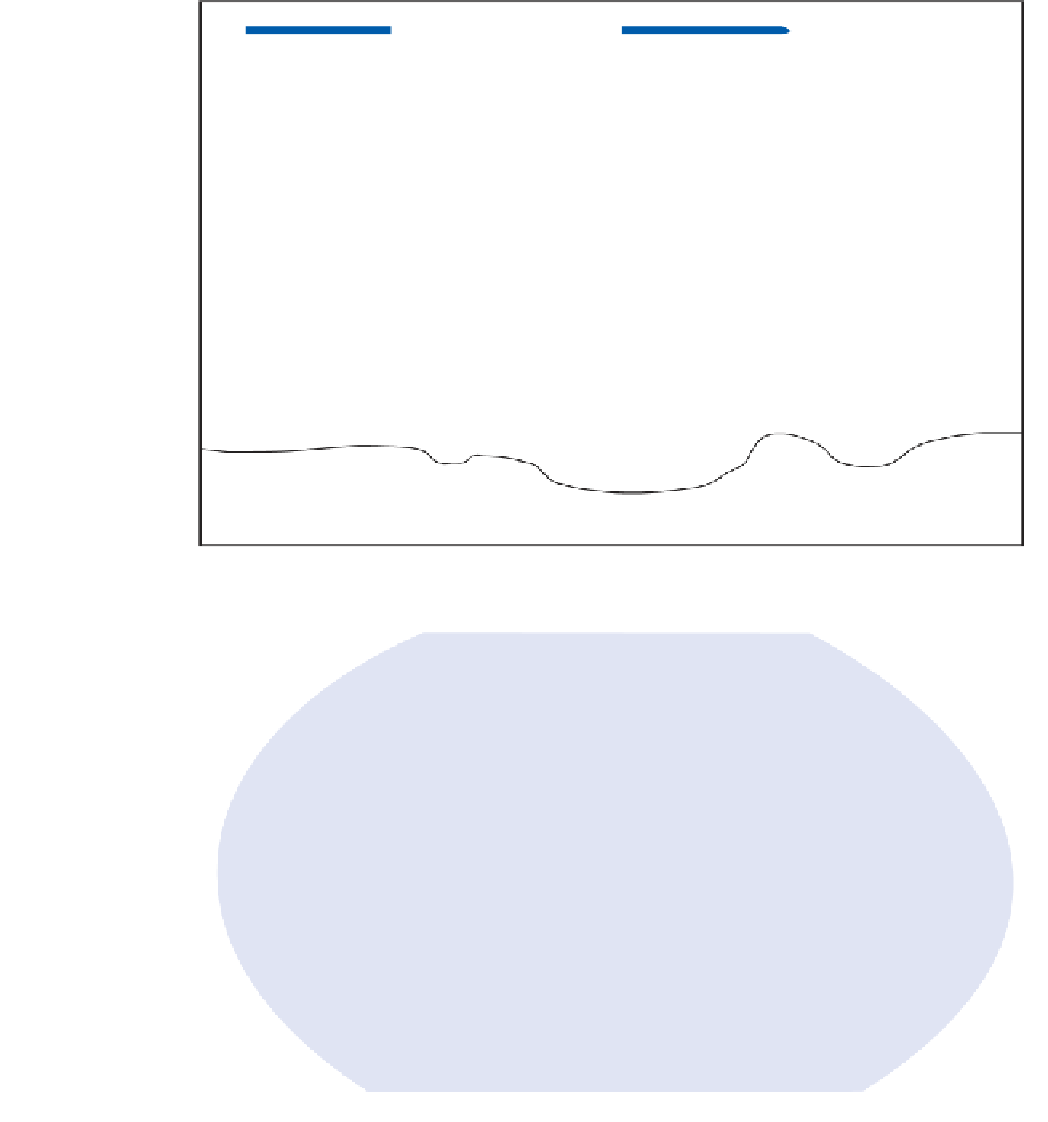
Figure 5.2
Schematic
representation of
the formation,
distribution and
impact of acid rain.
PREVAILING WINDS
Photochemical action
Photochemical action
Atmospheric moisture
OXIDATION
DISSOLUTION
SO
2
+H
2
O
H
2
SO
4
2H
+
+ SO
4
- -
H
+
+ NO
3
-
NO
X
+H
2
O
HNO
3
Wet deposition
SO
2
NO
X
NO
3
-
SO
4
- -
H
+
Dry deposition
NO
X
SO
2
pH 4.6
pH 7.0
Limestone
buffers acidity
Granite produces
acid-sensitive
soils and lakes
LONGITUDE
150
120
90
60
30
0
30
60
90
120
150
180
80
80
4.6
4.4 - 5.6
60
40
40
4.5 - 5
4 - 5
4 - 5.6
3.5
5.9
6.3
20
20
5.2
4.7
0
0
4.7
4.7
5.1
4.1
20
20
3.8 - 5.4
6.3
40
Estimated distribution of pH
The isolated values of pH from remote areas
Areas where effects have been reported
40
60
60
150
120
90
60
30
0
30
60
90
120
150
180