Geoscience Reference
In-Depth Information
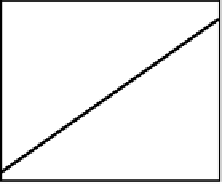
Figure 6.5.
A potassium-argon whole-rock isochron for tuffs
(volcanic ash) from the Olduvai Gorge in Tanzania. The age of these
rocks is very important because it is used to date both recent
geomagnetic reversals (as discussed in Section 3.2.1) and the early
hominoid remains which have been discovered in the gorge by
Leakey and co-workers. (After Fitch
et al
.(1976).)
5000
4000
3000
2000
1000
t = 2.04
±
0.02 Ma
0
1
2
3
4
x10
7
40
K
36
Ar
The non-radiogenic isotope
36
Ar is used to normalize this equation:
40
Ar
36
Ar
40
Ar
36
Ar
0
+
40
K
36
Ar
e
(
λ
A
+
λ
C
)
t
−
1
λ
A
λ
A
+
λ
C
now
=
(6.42)
now
An isochron can be constructed using this equation, which allows
t
and the initial
argon ratio to be estimated (Fig. 6.5). It is often possible to assume that the ini-
tial argon ratio [
40
Ar
36
Ar]
0
was 295.5, its present-day value in the atmosphere,
and so obtain an age from a single, whole rock or mineral, which makes this
an attractive dating method. In addition, the shorter half-life of
40
K and its rela-
tive abundance compared with the previously discussed elements mean that this
method is good for dating young rocks. However, problems can arise because
argon is a gas and thus easily lost from the system and because potassium is also
mobile. Another problem arises if the argon initially present in the sample was not
totally of atmospheric origin but incorporated some argon from crustal or mantle
outgassing: the calculated age would then be too great. Closure temperatures
(see Sections 6.2 and 7.8.5) for argon depend on the mineral involved, ranging
between approximately 100 and 500
◦
C; thus, each mineral gives a different age
(see Fig. 7.26 for an example). These differences in closure temperatures lead
to a potentially powerful method of unravelling the thermal history of the rocks
(discussed in Section 7.8.5).
/
6.7 Argon-argon
The three naturally occurring isotopes of argon are
36
Ar,
38
Ar and
40
Ar, which are
present in the atmosphere in proportions of 0.34%, 0.06% and 99.6%, respec-
tively. The argon-argon dating method depends first on bombarding a sample
with fast neutrons in a nuclear reactor, which converts some of the
39
K into
39
Ar.
The reaction is
39
K
+
n
39
Ar
+
p
→
(6.43)
where n is a neutron and p a proton. Following irradiation, the argon must be
extracted from the sample. Originally this was achieved by heating step by step
in a vacuum. Nowadays a laser is focussed to melt individual mineral grains. This
is termed the 'total-fusion' method. Alternatively 'laser-probe' dating releases