Geoscience Reference
In-Depth Information
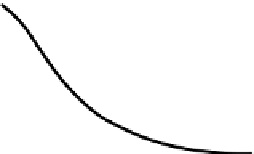
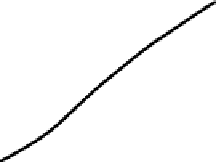
Figure 6.1.
The relationship between geochronological closure of a
mineral and its cooling history. The upper graph shows the cooling
history of a mineral. The vertical axis in the lower graph, the
diffusion coefficient
D
, approximates the rate of escape of the
radiogenic daughter product.
T
c
is the closure temperature, which is
attained at time
t
c
. Each mineral in a rock has a different set of
diffusion coefficients, resulting in a variety of closure temperatures
and times, as discussed in the text. (From Dodson (1973).)
T
c
t
c
Time
on the chemical and thermal history of the crust and mantle. Rubidium-strontium
dating is discussed first: it is simple and a good system to use to illustrate the
problems and pitfalls of geochronology.
6.3 Rubidium-strontium
For the decay of
87
Rb to
87
Sr, Eq. (6.11)is
[
87
Sr]
now
=
[
87
Rb]
now
(e
λ
t
−
1)
(6.26)
where [
87
Sr]
now
is the number of
87
Sr atoms and [
87
Rb]
now
is the number of
87
Rb
atoms, both measured now. Since strontium occurs naturally in rocks indepen-
dently of rubidium, it is not reasonable to assume that all the [
87
Sr]
now
is a result of
the decay of
87
Rb. Equation (6.26) must therefore be modified to include [
87
Sr]
0
,
the amount of originally occurring
87
Sr:
[
87
Sr]
now
=
[
87
Sr]
0
+
[
87
Rb]
now
(e
λ
t
−
1)
(6.27)
There are four isotopes of natural strontium with relative atomic masses 84, 86,
87 and 88, which have fractional abundances of about 0.6%, 10%, 7% and 83%,
respectively. Rubidium has just two isotopes,
85
Rb and
87
Rb;
87
Rb has a fractional
abundance of
28%.
Since strontium-86 is not a product of any radioactive decay, the amount of
strontium-86 present now should be the amount that was originally present:
∼
[
86
Sr]
now
=
[
86
Sr]
0
(6.28)