Geoscience Reference
In-Depth Information
exception was an anomalous event at
Lake Nyos,
Cameroon
.This crater lake sits above a pocket of
magma that leaks carbon dioxide into the lake water. On
August 21, 1986, a landside triggered a large release of
carbon dioxide that asphyxiated 1,700 people and 3,500
animals in nearby towns and villages.
Although direct health effects of CO
2
(g) have been
insignificant beyond the event at Lake Nyos, local
or global increases in CO
2
(g) increase temperatures
and water vapor. Both higher temperatures and higher
water vapor independently increase ozone and particu-
late matter, as discussed in Section 12.5.6. Ozone and
particulate matter both harm human health.
Table 3.8.
Sources and sinks of atmospheric carbon
monoxide
Sources
Sinks
Fossil fuel and biofuel
combustion
Atmospheric chemical
reaction to carbon dioxide
Biomass burning
Dissolution in surface water
Atmospheric chemical
reaction
Deposition to sea ice, snow,
soil, vegetation, and
structures
Plants and biological
activity in oceans
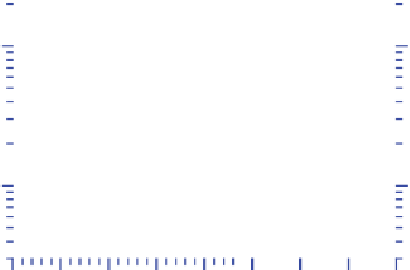
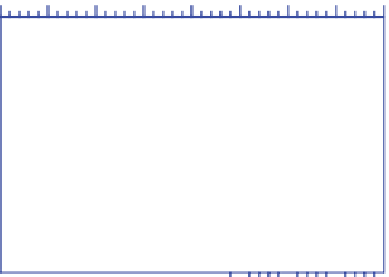
In 2008, about 70 million metric tonnes of CO(g)
were emitted from anthropogenic sources in the United
States (Figure 3.14). The largest source (73 percent) was
on-road plus nonroad transportation. Carbon monoxide
emissions decreased in the United States between 1970
and 2008 by about 62 percent, despite a large increase
in the number of vehicles. The reason was due primarily
to the development and mandatory use of the catalytic
converter in motor vehicles (Chapter 8).
3.6.3. Carbon Monoxide
Carbon monoxide
[CO(g)] is a tasteless, colorless, and
odorless gas. Although CO(g) is the most abundantly
emitted spatially and temporally varying gas aside from
H
2
O(g) and CO
2
(g), it plays a lesser role in ozone for-
mation in urban areas than do many organic gases. In
the background troposphere, however, it plays a rel-
atively larger role. CO(g) is a minor greenhouse gas
because it absorbs some thermal-IR radiation. Its emis-
sion and oxidation to CO
2
(g) also affect global climate.
CO(g) is not important with respect to stratospheric
ozone reduction or acid deposition. However, it is an
important component of urban and indoor air pollu-
tion because it has harmful short-term health effects.
It is one of six pollutants called
criteria air pollu-
tants
(Section 8.1.6) for which U.S. National Ambient
Air Quality Standards (NAAQS) were set by the U.S.
Environmental Protection Agency (U.S. EPA) under
the 1970 U.S. Clean Air Act Amendments (CAAA70).
CO(g) is regulated in most countries of the world today
(Section 8.2).
3.6.3.2. Mixing Ratios
Mixing ratios of CO(g) in polluted urban air away from
freeways are typically 2 to 10 ppmv. On freeways and in
traffic tunnels, they can exceed 100 ppmv. In indoor air,
hourly average mixing ratios can reach 6 to 12 ppmv
when a gas stove is turned on (Samet et al., 1987). In
the absence of indoor sources, CO(g) indoor mixing
ratios are usually less than are those outdoors (Jones,
100
3.6.3.1. Sources and Sinks
Table 3.8 summarizes the sources and sinks of CO(g).
Amajor source of CO(g) is incomplete combustion dur-
ing fossil fuel and biofuel combustion. CO(g) emission
sources include wildfires, biomass burning, nontrans-
portation combustion, some industrial processes, and
biological activity. Indoor sources of CO(g) include
water heaters, coal and gas heaters, and gas stoves. The
major sink of CO(g) is chemical conversion to CO
2
(g).
CO(g) is also lost by deposition to sea ice, snow, soil,
vegetation, and structures and dissolution in surface
water. Because it is relatively insoluble, its dissolution
rate is low.
Carbon monoxide
Nitrogen oxides
Reactive organic gases
Sulfur dioxide
Ammonia
PM
2.5
10
1970
1975
1980
1985
1990
1995
2000
2005
2010
Year
Figure 3.14.
U.S. anthropogenic emissions by
pollutant 1970-2008. Nitrogen oxides are NO
x
(g)
=
NO(g)
+
NO
2
(g). PM
2.5
is particulate matter
≤
2.5
m
in diameter. Data from U.S. EPA (2011a).



























































Search WWH ::

Custom Search