Geoscience Reference
In-Depth Information
(denoted by the double arrows) reaction
Reaction 3.17. The acid reacts with calcite, producing
the calcium ion and the bicarbonate ion by
CaCO
3
(s)
CO
2
(g)
CO
2
(aq)
(3.15)
+
+
CO
2
(g)
H
2
O(aq)
Gaseous
Dissolved
carbon
carbon
Calcium
Gaseous
Liquid
dioxide
dioxide
carbonate
carbon
water
dioxide
followed by the rapid combination of CO
2
(aq) with
water to form carbonic acid [H
2
CO
3
(aq)] and the dis-
sociation of carbonic acid to the
hydrogen ion
[H
+
],
the
bicarbonate ion
[HCO
3
−
], or the
carbonate ion
[CO
3
2
−
]bythereversible reactions
CO
2
(aq)
2HCO
3
Calcium Carbonic Calcium Biocarbonate
carbonate acid ion ion
(3.19)
Because Reaction 3.19 is reversible, it can proceed
either to the right or left. When the partial pressure
of CO
2
(g) is high, the reaction proceeds to the right,
breaking down calcite, removing CO
2
(g), and produc-
ing Ca
2
+
.Within soils, root and microorganism respira-
tion and organic matter decomposition cause the partial
pressure of CO
2
(g) to be about 10 to 100 times that in
the atmosphere (Brook et al., 1983). Thus, calcite is bro-
ken down, and CO
2
(g) is removed more readily within
soils than at soil surfaces. Dissolved calcium ultimately
flows with runoff back to the oceans, where some of it
is stored and the rest of it is converted to shell material.
Ca
2
+
CaCO
3
(s)
+
H
2
CO
3
(aq)
+
+
H
2
O(aq)
H
2
CO
3
(aq)
Dissolved
Liquid
Dissolved
carbon
water
carbonic
dioxide
acid
H
+
HCO
3
2H
+
CO
2
−
3
+
+
(3.16)
Hydrogen
Bicarbonate
Hydrogen
Carbonate
ion
ion
ion
ion
Ocean water is alkaline (or basic), the opposite of acidic
(Section 5.3.2.3), with a pH
8.1. Under such con-
ditions, nearly all dissolved CO
2
(g) dissociates to the
bicarbonate ion, and a small fraction dissociates to the
carbonate ion. Certain organisms in the ocean are able
to synthesize the carbonate ion with the calcium ion
[Ca
2
+
]toform calcium carbonate [CaCO
3
(s), calcite]
shells by
≈
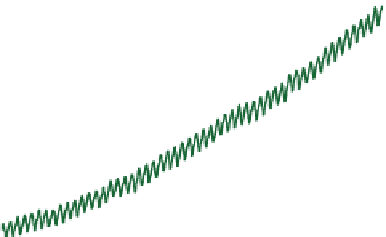
3.6.2.2. Mixing Ratios
Figure 3.11 shows that outdoor CO
2
(g) mixing ratios
have increased steadily since 1958 at the Mauna Loa
Observatory, Hawaii. Average global CO
2
(g) mixing
ratios have increased from approximately 275 ppmv in
the mid-1700s to approximately 393 ppmv in 2011. The
yearly increases are due to increased CO
2
(g) emission
from fossil fuel combustion and permanent deforesta-
tion resulting from biomass burning.
Ca
2
+
CO
2
−
3
+
→
CaCO
3
(s)
(3.17)
Calcium
Carbonate
Calcium
ion
ion
carbonate
When shelled organisms die, they sink to the bottom of
the ocean, where they are ultimately buried and their
shells are turned into calcite rock.
Another removal process of CO
2
(g) from the air is
chemical weathering
,which is the breakdown and
reformation of rocks and minerals at the atomic and
molecular level by chemical reaction. One chemical
weathering reaction is
400
380
CaSiO
3
(s)
+
CO
2
(g)
CaCO
3
(s)
+
SiO
2
(s)
(3.18)
360
Generic
Carbon
Calcium
Silicon
calcium
dioxide
carbonate
dioxide
silicate
(calcite)
(quartz)
340
in which calcium-bearing silicate rocks react with
CO
2
(g) to form calcium carbonate rock and quartz
rock [SiO
2
(s)]. At high temperatures, such as in the
Earth's mantle, the reverse reaction also occurs, releas-
ing CO
2
(g), which is expelled to the air by volcanic
eruptions.
Another chemical weathering reaction involves car-
bon dioxide and calcite rock. During this process,
CO
2
(g) enters surface water or groundwater by Reac-
tion 3.16 and forms carbonic acid [H
2
CO
3
(aq)] by
320
1960
1970
1980
1990
2000
2010
Year
Figure 3.11.
Yearly and seasonal fluctuations in
carbon dioxide mixing ratio at Mauna Loa
Observatory, Hawaii (19.4795
◦
N, 155.603
◦
W)
between 1958 and 2011. From Mauna Loa Data
Center (2011).













































































































Search WWH ::

Custom Search