Geoscience Reference
In-Depth Information
100
100
80
80
1 hPa (above 99.9%)
60
60
10 hPa (above 99%)
40
40
100 hPa (above 90%)
20
20
500 hPa (above 50%)
0
0
0
0.4
0.8
1.2
0
200
400
600
800
1000
Air density (kg m
-3
)
Air pressure (hPa)
(a)
(b)
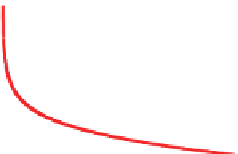
Figure 3.2.
(a) Pressure and (b) density versus altitude in the Earth's lower atmosphere. The pressure diagram
shows that 99.9 percent of the atmosphere lies below an altitude of about 48 km (1 hPa) and 50 percent lies
below about 5.5 km (500 hPa).
energy, the frictional loss, and thus heat production, is
low, so we feel cold. In the upper atmosphere, where
air density is low, the temperature can be extremely
high because each air molecule receives energy from
intense ultraviolet radiation and thus has a high kinetic
energy. However, we would feel cold if exposed to such
air because the air density is low, so few air molecules
would hit our skin, reducing the total heat production
on our skin. In sum, temperature is a measure of hotness
only at a given air density.
From gas kinetic theory, the absolute temperature (K)
of air is related to kinetic energy by
4
Measurements of relative air temperature changes
were first attempted in 1593 by Galileo, who devised
athermoscope to measure the expansion and contrac-
tion of air upon its heating and cooling, respectively.
However, the instrument did not have a scale and was
unreliable. In the mid-seventeenth century, the thermo-
scope was replaced by the liquid-in-glass thermometer
developed in Florence, Italy. In the early eighteenth
century, useful thermometer scales were developed by
Gabriel Daniel Fahrenheit
(1686-1736) of Germany
and
Anders Celsius
(1701-1744) of Sweden.
The temperature at a given location and time is
affected by energy transfer processes, including con-
duction, convection, advection, and radiation. These
processes are discussed briefly here.
1
2
M
¯
2
a
=
v
k
B
T
(3.1)
×
10
−
23
kg
where
k
B
is
Boltzmann's constant
(1.3807
M
is the average mass of one air
m
2
s
−
2
K
−
1
molec
−
1
),
10
−
26
kg molec
−
1
), and ¯
molecule (4.8096
a
is the
average
thermal speed
of an air molecule (m s
−
1
). The
right side of Equation 3.1 is the kinetic energy of an air
molecule at its average thermal speed.
×
3.2.1. Conduction
Conduction
is the transfer of energy in a medium
(the conductor) from one molecule to the next in the
presence of a temperature gradient. The medium, as a
whole, experiences no molecular movement. Conduc-
tion occurs through soil, air, and particles. Conduction
affects air temperature by transferring energy between
the soil surface and the bottom molecular layers of
the air. The rate of a material's conduction is deter-
mined by its
thermal conductivity
(
Example 3.2
What is the thermal speed of an air molecule at
200 K? At 300 K?
Solution
From Equation 3.1, the thermal speed of an air
molecule is 382 m s
−1
at 200 K and 468 m s
−1
at
300 K.
,Jm
−
1
s
−
1
K
−
1
),
which quantifies the rate of flow of thermal energy
through a material in the presence of a temperature gra-
dient. Table 3.1 gives thermal conductivities of a few


















Search WWH ::

Custom Search