Geoscience Reference
In-Depth Information
Table 1.5.
Structures of some common organic compounds found in air
Alkane
Alkene
Cycloalkene
Hemiterpene
Ethane
Ethene
Cyclopentene
Isoprene
C
2
H
6
(g)
C
2
H
4
(g)
C
5
H
8
(g)
C
5
H
8
(g)
H
H
C
H
H
CH
3
H
CC
HC
C
H
H
2
C
CH
CC
H
H
H
H
2
CCH
2
H
2
C
CH
2
H
Aromatic
Alcohol
Aldehyde
Ketone
Toluene
Methanol
Formaldehyde
Acetone
C
6
H
5
CH
3
(g)
CH
3
OH(g)
HCHO(g)
CH
3
COCH
3
(g)
O
CH
3
H
O
H
H
H
HC
HC
C
C
H
HC
O
H
H
H
H
both C and H, but may also contain other elements.
Methane is the simplest organic compound.
Organic compounds that contain only H and C
are
hydrocarbons
.Hydrocarbons include alkanes,
cycloalkanes, alkenes, cycloalkenes, alkynes, aromat-
ics, and terpenes. Examples of some of these groups
are given in Table 1.5.
Alkanes
(paraffins) are open-
chain (noncyclical) hydrocarbons with a single bond
between each pair of carbon atoms and the molecular
formula C
n
H
2
n
+
2
.
Cycloalkanes
(not shown) are sim-
ilar to alkanes, but with a cyclical structure.
Alkenes
(olefins) are open-chain hydrocarbons with a double
bond between one pair of carbon atoms and the molecu-
lar formula C
n
H
2
n
.
Cycloalkenes
are similar to alkenes,
but with a cyclical structure.
Alkynes
(acetylenes, not
shown) are open-chain hydrocarbons with a triple bond
between at least one pair of carbon atoms.
Te rpenes
are a class of naturally occurring hydrocarbons that
include hemiterpenes (C
5
H
8
),
monoterpenes
(C
10
H
16
),
sesquiterpenes
(C
15
H
24
),
diterpenes
(C
20
H
32
), and so
on.
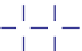
Aromatic hydrocarbons
are hydrocarbons with a
benzene ring and possibly other H and C atoms attached
to the ring. Two representations of a benzene ring are
shown in Figure 1.24.
Aromatics are so named because the first aromat-
ics isolated were obtained from substances that had a
pleasant fragrance, or aroma. Around 1868, Austrian
chemist
Joseph Loschmidt
(1821-1895) found that
such aromatic compounds could be obtained by replac-
ing one or more hydrogen atoms on a benzene ring
with another atom or group. The name aromatic was
subsequently applied to any compound that had a
benzene ring in its structure. Loschmidt was the first
to explain the structure of benzene, toluene, and ozone.
He is also the first to quantify accurately Avogadro's
number (Section 3.4).
When methane, a slowly reacting hydrocarbon, is
excluded from the list of hydrocarbons, the remaining
hydrocarbons are called
nonmethane hydrocarbons
(NMHCs)
.When oxygenated functional groups, such
as aldehydes, ketones, alcohols, acids, and nitrates, are
added to hydrocarbons, the resulting compounds are
oxygenated hydrocarbons
.InTable 1.5, the alcohol,
aldehyde, and ketone are oxygenated hydrocarbons.
Nonmethane hydrocarbons and oxygenated hydrocar-
bons are
reactive organic gases (ROGs)
.
To tal organic
gas (TOG)
is the sum of ROGs and methane.
Vo l a tile
organic compounds (VOCs)
are organic compounds
with relatively low boiling points that, therefore, read-
ily evaporate. Although all VOCs are not necessar-
ily ROGs, these terms are often interchanged. Finally,
aldehydes and ketones are called
carbonyls
.Thesum
of nonmethane hydrocarbons and carbonyls is
non-
methane organic carbon (NMOC)
.
C
HC
CH
HC
CH
H
Figure 1.24.
Two representations of benzene ring.























Search WWH ::

Custom Search