Geoscience Reference
In-Depth Information
400
1979
1999
2005
2010
40
O
3
(ppmv)
350
30
300
20
250
O
3
(molecules cm
-3
×
10
-12
)
Zonal and yearly average
Air (molecules cm
-3
×
5
×
10
-19
)
10
200
-90
-60
-30
0
30
60
90
Latitude (degrees)
0
02468 012
14
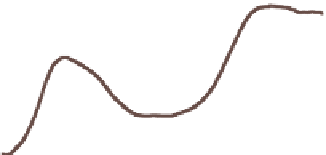
Figure 11.3.
Variation of yearly and zonally averaged
ozone column abundance with latitude during 1979,
1999, 2005, and 2010. Data were obtained from the
satellite-based Total Ozone Mapping Spectrometer
(TOMS) and made available by NASA Goddard Space
Flight Center, Greenbelt, Maryland.
Figure 11.4.
Example vertical variation in ozone
mixing ratio, ozone number concentration, and air
number concentration with altitude. In this example,
the ozone mixing ratio at the surface is 0.20 ppmv,
the level of a Stage 1 smog alert in the United States.
The polar vortex prevents outside stratospheric air
from entering the Antarctic region and Antarctic air
from escaping.
ASHspringminimum of less than 150 DUs over
the South Pole due to chemical reactions of chlorine
and bromine radicals with ozone. This minimum is
the
Antarctic ozone hole
,which is defined as the
area of the Antarctic over which the ozone column
abundance decreases below 220 DUs. The minimum
column abundance at a specific location (not zonally
averaged) within the ozone hole can drop to 80 DUs.
Figure 11.4 shows a typical variation of ozone mixing
ratio, ozone number concentration, and total air number
concentration with altitude. The ozone number concen-
tration (molecules of ozone per cubic centimeter of air)
in the stratosphere generally peaks at 25 to 32 km in
altitude. The ozone mixing ratio (number concentration
of ozone divided by that of dry air) peaks at a higher
altitude than does the number concentration. The peak
ozone number concentration in the stratosphere is close
to that in polluted urban air. The
peak ozone mixing
ratio in the stratosphere
(near 10 ppmv), however,
is much higher than is that in very polluted urban air
(0.2-0.35 ppmv) or free-tropospheric air (20-40 ppbv).
Figure 11.3 shows the variation with latitude of the
zonally and yearly averaged ozone column abundance
in 1979, 1999, 2005, and 2010. The ozone layer was
thin near the Equator for all 4 years. In 1999, the ozone
column abundance between 15
◦
S and 15
◦
Nincreased
compared with 1979. Such increases, relative to 1979
ozone, occurred in about one-third of the years between
1979 and 2010.
The yearly averaged ozone column abundance 60
◦
S
to 90
◦
Sisalways greater than over the Equator; how-
ever, since 1979, ozone 60
◦
Sto90
◦
S has declined due
to the seasonal Antarctic ozone hole (Section 11.4). The
decline held steady from 1999 to 2010.
The yearly averaged ozone column abundance 60
◦
N
to 90
◦
Nisalso greater than over the Equator. For most
years since 1979, the column abundance 60
◦
Nto90
◦
N
has been lower than in 1979. Two exceptions were dur-
ing 1999 and 2010, when the column abundance was
nearly the same as in 1979. When a reduction over
the Arctic occurs, it is called an
Arctic ozone dent
(Section 11.4).
11.2. Relationship between the Ozone
Layer and Ultraviolet Radiation
The ozone layer prevents damaging UV wavelengths
from reaching the Earth's surface. The UV portion of
the solar spectrum is divided into far- and near-UV
wavelengths (Figure 2.5). Near-UV wavelengths are
further divided into UV-A, -B, and -C wavelengths
(Section 2.2). Gases, particularly ozone and oxygen,
and aerosol particles absorb most UV radiation before
it reaches the Earth's surface. Decreases in stratospheric
ozone increase the transmission of UV to the surface.
Enhancements in UV at the surface damage life. In
this subsection, processes affecting UV radiation are
summarized.
Figure 11.5 shows the intensity of downward UV and
visible radiation at the top of the atmosphere (TOA) and
at the ground. Of the incident solar radiation at the TOA,
only wavelengths longer than 0.29
m penetrate to the




































































Search WWH ::

Custom Search