Geoscience Reference
In-Depth Information
Figure 9.1.
Antoine Henri Becquerel (1871-1937).
Edgar Fahs Smith Collection, University of
Pennsylvania Library.
Figure 9.2.
Ernest Rutherford (1871-1937). American
Institute of Physics Emilio Segr `eVisualArchives,
William G. Myers Collection.
234
Pa decays further to uranium-234 (
234
U), then to
thorium-230 (
230
Th), then to radium-226 (
226
Ra), and
then to radon-222 (
222
Rn).
Whereas radon precursors are bound in minerals
(Lyman, 1997),
222
Rn is a gas and can escape through
beta particles. Rutherford later discovered the gamma
ray as well.
Equation 9.1 summarizes the radioactive decay path-
way of
238
Uto
206
Pb. Numbers shown are half-lives of
each decay process.
(9.1)
When it decays to produce radon,
238
Ufirstreleases
an alpha particle, producing thorium-234 (
234
Th), which
decays to protactinium-234 (
234
Pa), releasing a beta par-
ticle.
234
Pa has the same number of protons and neutrons
in its nucleus as does
234
Th, but
234
Pa has one less elec-
tron than does
soil and unsealed floors into houses, where its mixing
ratio builds up in the absence of ventilation.
222
Rn has a
half-life of 3.8 days. It decays to polonium-218 (
218
Po),
which has a half-life of 3 minutes and decays to lead-214
(
214
Pb).
218
Po and
214
Pb, referred to as
radon progeny
,
are electrically charged and can be inhaled or attach to
234
Th, giving
234
Pa a positive charge.

































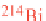






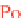

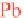

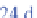




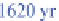

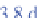








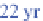

































Search WWH ::

Custom Search