Geoscience Reference
In-Depth Information
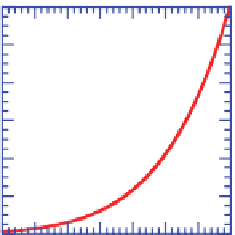
The most abundant condensing gas in the air is water
vapor. Figure 5.12a shows the SVP of water over a liq-
uid surface versus temperature. It indicates that the SVP
of water vapor increases superlinearly with increasing
temperature. This rule applies to any gas. The con-
sequence is that
gases evaporate faster with increas-
ing temperature and condense faster with decreasing
temperature
.
Figure 5.12b shows the SVP of water over a liquid
surface and an ice surface at temperatures below 0
◦
C.
Because water vapor's partial pressure cannot exceed
its SVP without the excess vapor condensing, the SVP
is effectively the maximum possible partial pressure of
water vapor in the air at a given temperature. Near the
poles, where temperatures are below 0
◦
C, the SVP can
be as low as 0.0003 percent of sea level air pressure.
Near the Equator, where temperatures are close to 30
◦
C,
the SVP can increase to 4 percent or more of sea level
air pressure.
Condensation
Evaporation
Gas
Gas
Particle
Particle
(a)
(b)
Figure 5.11.
(a) Condensation occurs when the partial
pressure of a gas away from a particle surface
(represented by the thick cloud of gas away from the
surface) exceeds the saturation vapor pressure (SVP)
of the gas over the surface (represented by the thin
cloud of gas near the surface). (b) Evaporation occurs
when the SVP exceeds the partial pressure of the gas.
The schematics are not to scale.
to gas). The partial pressure of the gas immediately
over the particle's surface is called the gas's
satura-
tion vapor pressure
(SVP). If the partial pressure of
the gas away from the surface increases above the SVP
overthe surface, excess molecules diffuse to the surface
(Figure 5.11a) and condense. If the gas's partial pres-
sure decreases below the SVP, gas molecules over the
surface diffuse away from the surface (Figure 5.11b),
and liquid molecules on the surface evaporate to main-
tain saturation over the surface. In sum,
if the ambient
partial pressure of a gas exceeds the gas's SVP, conden-
sation occurs
.Iftheambient partial pressure of the gas
falls below the gas's SVP, evaporation occurs. Thus, the
lower its SVP, the more likely a gas is to condense.
Example 5.2
Determine the maximum partial pressure and
percentage water vapor in the atmosphere at 0
◦
C
and 30
◦
C.
Solution
From Figure 5.12a, the SVP and, therefore, the
maximum partial pressure of water vapor at 0
◦
C
and 30
◦
Care6.1and 42.5 hPa, respectively.
Because sea level dry air pressure is 1,013 hPa,
water vapor comprises no more than 0.6 and 4.2
percent of total air by volume, respectively, at
these two temperatures.
120
8
7
100
6
80
5
60
4
Over liquid
water
Over liquid
water
3
40
2
20
1
Over ice
0
0
-20 -10
0
10
20
30
40
50
-50
-40
-30
-20
-10
0
Temperature (
o
C)
Temperature (
o
C)
(a)
(b)
Figure 5.12.
Saturation vapor pressure over (a) liquid water versus temperature and (b) liquid water and ice
versus temperature.




























Search WWH ::

Custom Search