Geoscience Reference
In-Depth Information
methane) in the presence of sunlight. The most recog-
nized gas-phase by-product of smog reactions is ozone
because ozone has harmful health effects (Section 3.6.5)
and is an indicator of the presence of other pollutants.
On a typical day, ozone forms following the emission
of NO(g) and ROGs. Emitted pollutants are called
pri-
mary pollutants
. Pollutants, such as ozone, that form
chemically or physically in the air are called
secondary
pollutants
.
Primary pollutant ROGs are broken down by chem-
ical reaction into
peroxy radicals
, denoted by RO
2
(g).
Peroxy radicals and NO(g) form secondary pollutant
ozone by the following sequence:
NO(g)
Nitric
oxide
The plot also shows that, for low ROGs, increases in
NO
x
(g) above 0.05 ppmv decrease ozone. For high
ROGs, increases in NO
x
(g) always increase ozone.
The plot is useful for regulatory control of ozone. If
ROG mixing ratios are high (e.g., 2 ppmC) and NO
x
(g)
mixing ratios are moderate (e.g., 0.06 ppmv), the plot
indicates that the most effective way to reduce ozone
is to reduce NO
x
(g). Reducing ROGs under these con-
ditions has little effect on ozone. If ROG mixing ratios
are low (e.g., 0.7 ppmC) and NO
x
(g) mixing ratios are
high (e.g., 0.2 ppmv), the most effective way to reduce
ozone is to reduce ROGs. Reducing NO
x
(g) under these
conditions actually increases ozone. In many polluted
urban areas, the ROG:NO
x
(g) ratio is lower than 8:1,
indicating that limiting ROG emissions should be the
most effective method of controlling ozone. However,
because ozone mixing ratios depend not only on chem-
istry, but also on meteorology, deposition, and gas-to-
particle conversion, such a conclusion is not always
clear cut.
Figure 4.13 shows the evolution of NO(g), NO
2
(g),
and O
3
(g) during one day at two locations - central
Los Angeles and San Bernardino - in the Los Angeles
Basin. In the basin, a daily sea breeze transfers primary
pollutants [NO(g) and ROGs], emitted on the west side
of the basin (i.e., central Los Angeles) to the east side
of the basin (i.e., San Bernardino), where they arrive as
secondary pollutants [O
3
(g) and PAN]. Whereas NO(g)
mixing ratios peak on the west side of Los Angeles, as
shown in Figure 4.13a, O
3
(g) mixing ratios peak on the
east side, as shown in Figure 4.13b. Thus, the west side
of the basin is a
source region
, and the east side is a
receptor region
of photochemical smog.
Asignificant difference between ozone production in
urban air and the background troposphere is that peroxy
radicals convert NO(g) to NO
2
(g) in urban air but less
so in clean air. As a result, the NO
2
(g):NO(g) ratio is
much higher in urban air than clean air. Reaction 4.4
suggests that an increase in the NO
2
(g):NO(g) ratio
increases ozone levels above the pure photostationary-
state ozone level.
R O
2
(g)
Organic
peroxy
radical
NO
2
(g)
Nitrogen
dioxide
R O(g)
Organic
oxy
radical
+
→
+
(4.37)
NO(g)
Nitric
oxide
NO
2
(g)
Nitrogen
dioxide
+
O
3
(g)
Ozone
→
+
O
2
(g)
Molecular
oxygen
(4.38)
NO
2
(g)
Nitrogen
dioxide
NO(g)
Nitric
oxide
O(g)
Atomic
oxygen
+
h
→
+·
<
420 nm
(4.39)
M
→
O(g)
Atomic
oxygen
·
+
O
2
(g)
Molecular
oxygen
O
3
(g)
Ozone
(4.40)
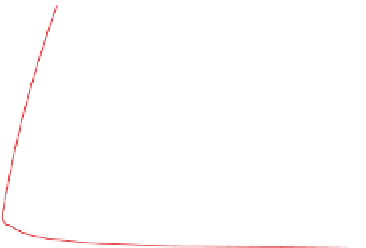
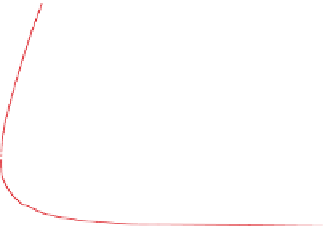
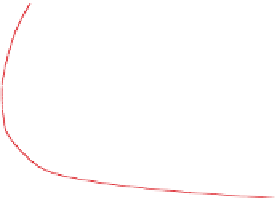
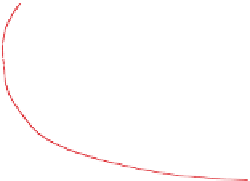
Figure 4.12 shows ozone mixing ratios resulting from
different initial mixtures of NO
x
(g) and ROGs. This
plot is called an
ozone isopleth
.The figure shows that,
for low mixing ratios of NO
x
(g), ozone mixing ratios
are relatively insensitive to the quantity of ROGs. For
high NO
x
(g), an increase in ROGs increases ozone.
0.25
0.4
0.2
0.15
0.1
0.05
4.3.1. Emissions of Photochemical
Smog Precursors
Major gases emitted in urban air include nitrogen
oxides, ROGs, carbon monoxide [CO(g)], and sulfur
oxides [SO
x
(g)
0
0
0.5
1
1.5
2
ROG (ppmC)
Figure 4.12.
Peak ozone mixing ratios resulting from
different initial mixing ratios of NO
x
(g) and reactive
organic gases (ROGs). The ROG:NO
x
(g) ratio along
the line through zero is 8:1. Adapted from
Finlayson-Pitts and Pitts (1999).
SO
3
(g)]. Of these, NO
x
(g)
and ROGs are the main precursors of photochemi-
cal smog. Sources of CO(g), SO
x
(g), and NO
x
(g) are
primarily incomplete combustion. Sources of ROGs
=
SO
2
(g)
+











Search WWH ::

Custom Search