Geoscience Reference
In-Depth Information
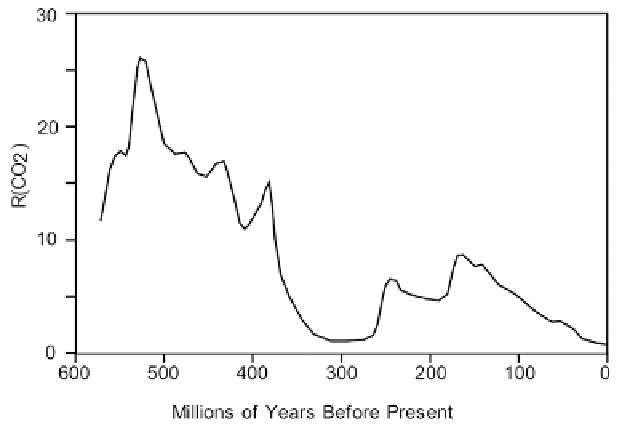
Figure 2.30. Plot of R(CO
2
) vs. time based on geological models (adapted from Berner, 2004).
exchanged between the oceans and atmosphere, and dissolved organic matter is
carried in solution by rivers from soils to the sea.''
As the short-term cycle proceeds, concentrations of the two principal
greenhouse gases, CO
2
and CH
4
, can change as a result of perturbations of the
cycle, resulting in global warming and cooling over centuries and many millennia.
Over longer periods of time (millions of years) additional processes can add or
remove CO
2
. Because there is more than a thousand times more carbon in rocks
than there is in the oceans, atmosphere, biosphere, and soils combined, carbon
transfers to and from rocks can result in significant changes in atmospheric CO
2
over long time periods. Berner (2004) discussed the processes whereby carbon is
exchanged with rocks. Two opposing processes are involved. CO
2
is stored in
rocks as calcium carbonate. Decarbonization via volcanism, metamorphism, and
diagenesis, releasing CO
2
to the atmosphere while producing calcium silicate.
Berner (2004) described how these cycles operated over the past 550 million years.
The details are extensive and well beyond the scope of this review.
Using geological models, estimates have been made of the concentration of
Berner (2004):
''The most dramatic feature of the curve is the large drop in CO
2
occurring in
the mid-Paleozoic (400-300Ma). This drop is due mainly to a combination of
changes brought about by the rise of large vascular land plants. The plants both
accelerated weathering and provided biologically resistant organic remains for
burial in sediments, causing a drop in CO
2
.''
Search WWH ::

Custom Search