Geoscience Reference
In-Depth Information
FMQ
2Fe
3
O
4
+
3SiO
2
)
3Fe
2
SiO
4
+
O
2
a)
:
-
6
Mt
60
Mt
40
Fe mainly in
oxide minerals
Mt
80
Magnetite (M) + Quartz (Q)
)
Fayalite (F) + Oxygen
-
8
Mt
100
At very high fo
2
, iron occurs in its ferric state, mainly in
haematite, as shown by the reaction (MH):
10
-
Mt
20
-
12
MH
6Fe
2
O
3
)
4Fe
3
O
4
O
2
:
+
-
14
Haematite (H)
)
Magnetite (M) + Oxygen
-
16
For the system Fe
SiO
2
the reactions FMQ and QIF
mark the fo
2
limits of fayalite, and MH the upper fo
2
limit
for the stability of magnetite. Consequently, fo
2
is a useful
guide to whether the iron occurs in silicates, and if not, in
which iron oxide.
Magnesium and iron can substitute for each other in a
variety of silicates. When magnesium substitutes for iron in
silicates it stabilises them to higher fo
2
, i.e. the FMQ is shifted
upwards decreasing the stability field of titanomagnetites. If
they contain enough magnesium, iron silicates, containing
ferrous iron, may be stable even in the presence of haematite.
The presence of titanium makes these species more stable
relative to silicates than their iron-only end-members.
Ferrous iron substituting for ferric iron has the same effect.
In summary, in a mineral assemblage containing sili-
cates and oxides, the Fe/Mg ratio of the silicates, the titan-
ium contents, ferrous/ferric ratios of the oxides, and
oxygen fugacities are all inter-related. However, fo
2
is not
a control imposed upon the system, but rather is governed
by the composition of the primary melt (if the rock is
igneous) and by the mineral reactions that occurred during
the formation of the rock. Oxygen is extremely rare in the
geological environment (except at, or very near, the Earth
-
O
-
-
18
Fe mainly in
silicate minerals
-
20
-
22
-
24
500
600
700
800
900
1000
1100
1200
Temperature (°C)
b)
-
6
Mt
60
Mt
40
Mt
80
Fe mainly in
oxide minerals
-
8
Mt
100
-
10
Mt
20
-
12
-
14
-
16
-
18
Fe mainly in
silicate minerals
-
20
-
22
-
24
500
600
700
800
900
1000
1100
1200
'
s
surface). Therefore, a reaction such as the oxidation of
fayalite to magnetite can only take place in association with
some other reaction that is a source of oxygen.
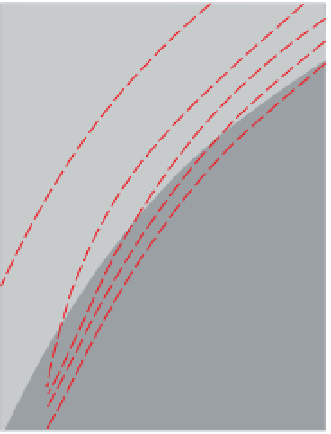
Figure 3.44
shows estimates of temperature
Temperature (°C)
c)
-
6
Mt
60
Mt
40
Mt
80
Fe mainly in
oxide minerals
-
8
fo
2
condi-
tions for various types of igneous rocks, along with selected
titanomagnetite and titanohaematite curves. The curves
are for solid-solution pairs that are in equilibrium. Of
course, this is unlikely to be the case in reality, especially
-
Mt
100
-
10
Mt
20
-
12
-
14
-
16
-
18
Figure 3.44
Temperature, oxygen fugacity and silica-content
controls on magnetic mineral oxides; (a) basic extrusive suites,
(b) intermediate extrusive suites and (c) acid extrusive suites. The
heavy line is the fayalite
Fe mainly in
silicate minerals
-
20
quartz buffer curve at 10
5
Pa.
The dashed lines represent experimentally coequilibrated
ulvospinel
-
magnetite
-
-
22
-
24
-
500
600
700
800
900
1000
1100
1200
Temperature (°C)


































































































































































































































































































































Search WWH ::

Custom Search