Geoscience Reference
In-Depth Information
3.9.3
Magnetism of igneous rocks
Königsberger ratio (Q)
10
-1
10
0
10
1
10
2
10
3
There are a large number of chemical and physical factors
involved in the formation of an igneous mass which influ-
ence its magnetic properties. These factors include: the bulk
composition of the magma, the presence of biotite which
can control the amount of magnetite produced, the rate of
cooling, order of mineral fractionation, composition of the
magmatic gases, and access to the atmosphere or the ocean.
These factors primarily affect whether magnetic minerals
are formed, and also determine the secondary controls on
magnetism such as the size, shape and orientation of the
magnetic mineral grains. All these factors, plus of course
the abundance of magnetic minerals, can vary throughout
the igneous mass, producing large variations in magnetic
properties (McEnroe et al.,
2009
). Even within individual
flows, magnetic properties may vary signi
cantly owing to
the effects of subsolidus exsolution and deuteric oxidisation
of primary iron oxides related to variations in cooling rates;
the effects associated with the almost ubiquitous low-
temperature alteration found in igneous terrains.
Given the many factors involved, only a general descrip-
tion of the magnetic properties of the different igneous
rock types is presented here, and there are nearly always
exceptions. Magnetite is mainly considered in the descrip-
tion because it is a common constituent of igneous rocks,
pyrrhotite much less so.
Basalt/dolerite
Gabbro/norite
Spilites
Dykes/sills
Flows
Pillow lavas
Felsic volcanics
Andesite & intermediate volcanics
Granite/granodiorite/tonalite
Diorite/monzonite/syenite
Peridotite including serpentinised dunite
Kimberlite
Sediments/metasediments
Mg-bearing
Haem-bearing
Meta-igneous
Iron formation
Laterite
Pyrrhotite-bearing rocks
& mineralisation
Massive Po
Disseminated Po
Skarn
Mg skarn
Po skarn
Induced
magnetism
dominant
Remanent
magnetism
dominant
Figure 3.43
Ranges in Königsberger ratio for the common rock types.
The darker shading indicates the most common parts of the ranges.
particularly high values often being associated with
pyrrhotite-bearing rocks and pillow lavas. The strength of
remanent magnetism, and the Königsberger ratio, also
reflects magnetic mineral content, although grain size and
grain microstructure are also important influences. Despite
this and the wide variability of magnetic properties, some
important magnetic relationships can be identified between
and within different rock classes and geological environ-
ments. Nevertheless, the fundamental problem remains that
the relationship between rock magnetism and geological
processes is not fully understood for all geological processes
and environments. When a particular aspect of rock mag-
netism is studied in detail, the relationship is almost invari-
ably shown to be complex. Much work remains to be done
to further our understanding of magnetic petrology.
We discuss the large and complex subject of magnetic
petrophysics in terms of the various rock classes and dif-
ferent geological environments and processes. It is imprac-
tical to provide a comprehensive description of the subject
in the space available to us, and inevitably there will be
exceptions made to the generalisations below. Important
Grant (
1984
).
3.9.3.1
Formation of magnetic iron oxides
Iron (Fe) occurs in a range of oxidation states in the
natural environment, the most common being ferrous
(Fe
2+
) and ferric (Fe
3+
) iron. The oxidation state of iron
chemical system without Ti or Mg, when fo
2
is very low,
such as occurs in the Earth
'
s core, iron may occur as a
metal (Fe
0
). At higher fo
2
, in a silicate-bearing system
ferrous iron occurs and is mostly incorporated into para-
magnetic silicate minerals like fayalite. The reaction (QIF)
is (written with the high-entropy side to the right, as are
others in this section):
2Fe
0
QIF
Fe
2
SiO
4
)
SiO
2
O
2
:
+
+
Fayalite (F)
)
Iron (I) + Quartz (Q) + Oxygen
At increasingly higher fo
2
, iron is present in either its
ferrous or ferric states, and is mostly incorporated into
magnetite. The change is described by the reaction (FMQ):
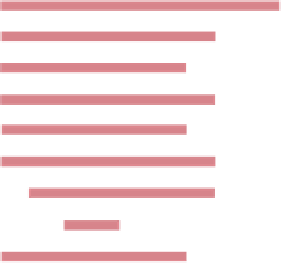








































Search WWH ::

Custom Search