Geoscience Reference
In-Depth Information
450
400
350
300
250
300
350
400
450
500
550
600
650
700
Emission Wavelength (nm)
450
400
350
300
300
350
400
450
500
550
600
650
700
Emission Wavelength (nm)
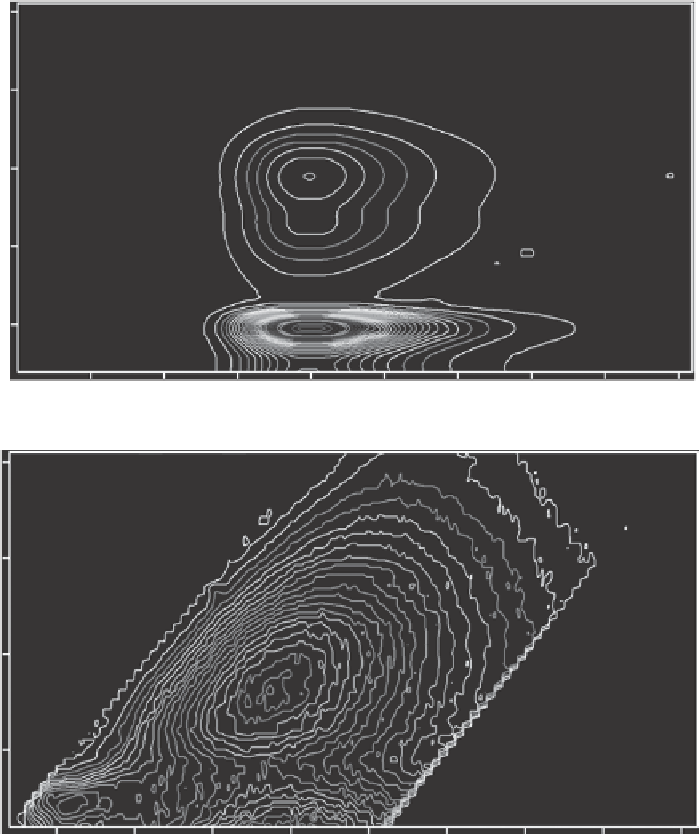
Figure 3.1. EEMs for quinine bisulfate (left) and Columbia River water (right) shown in contour (top)
and three-dimensional views (bottom).
of aquatic humic substances and is attributed to several factors, perhaps the most important
of which is that CDOM is composed of a mixture of individual fluorophores. It is likely
that some of these are families of compounds that share a common fluorophore backbone
but have different ring substitutions, giving them slightly different excitation and emission
maxima. In addition, charge transfer reactions between compounds may explain the near
continuum of excitation and emission in bulk water samples (Del Vecchio and Blough,
2004
). Peak A
C
has a wide range of emission but a narrow range of excitation. In the envi-
ronment, peaks C and A
C
are always observed together, although the ratio of the two peaks