Geoscience Reference
In-Depth Information
E
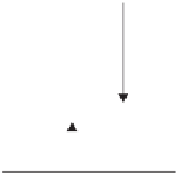
(a) Singlet Ground State
S = 0
Mutiplicity (2S+1) = 1
(b) Singlet Excited State
S = 0
Mutiplicity (2S+1) = 1
(3) Singlet Ground State
S = 1
Mutiplicity (2S+1) = 3
Figure 1.4. The relationship between spin orientation and multiplicity for electrons in singlet and
triplet states. The direction of the arrows indicates the orientation of each electron or spin quantum
number (ms) with values of either +½ or -½.
singlet state (Lakowitz,
2006
). This is illustrated in
Figure 1.3
and explained further in
Section 1.3.2.2
.
In generic terms a molecular energy state is the sum of the electronic, vibrational, rota-
tional, nuclear, and translational components within a molecule. The energy required to
change the distribution of an electron within in a molecular orbital is on the order of several
electron volts. As a consequence, photons emitted or absorbed when such changes occur lie
in the visible and ultraviolet regions (
Table 1.1
). In some cases the relocation of an electron
may be so extensive that it results in the breaking of a bond and the dissociation of the mol-
ecule, as is the case in many photochemical reactions. For most organic molecules the low-
est energy state, that is, the ground state, contains electrons that are spin-paired (
electronic
singlet
state). Under standard temperature and pressure most molecules have only enough
intrinsic energy to exist in the lowest vibrational level of the ground state. Therefore, exci-
tation for these molecules originates from this vibrational level of the ground state. Before
we examine the fluorescence process in detail it is important first to examine the interaction
of energy and electronic states more closely.
1.3.1.2 Absorption
A prerequisite to fluorescence is absorption, so it is first important to understand some of
the processes that occur during absorption. When a molecule
absorbs
radiation it's energy
is increased (Lakowitz,
2006
, chapter 2). This increase energy corresponds to the energy of
the absorbed photon and can be expressed by
Eq. (1.5)
:
Eh
hc
==
ν
λ
(1.5)
where
h
is Planck's constant, nu (
ν
) and lambda (
λ
) are the frequency and the wavelength of
the radiation respectively, and
c
is the velocity of light. The change in energy of a molecule