Geoscience Reference
In-Depth Information
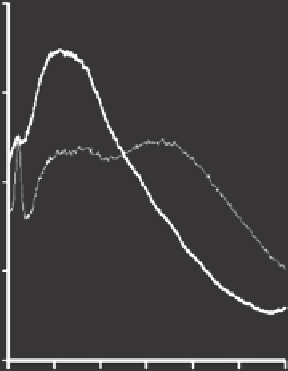
0.4
0.3
0.2
0.1
0
300
340 380
Wavelength (nm)
420 460 500 540
Figure 3.12. Typical fluorescence spectra of untreated (black line) and treated wastewater (gray line)
using 280 nm excitation.
combinations, for the removal of organic matter from different wastewaters. In this study,
fluorescence was employed because of its specificity in measuring humic substances, aro-
matic compounds, and heterocyclic systems. Rather than obtaining fluorescence emission
spectra, specific intensities at 490 nm were obtained using a fixed excitation wavelength
of 365 nm. In addition to these fluorescence measurements the UV absorbance at 280 nm
was also measured, and correlated with respective chemical oxygen demand (COD) values.
The COD provides an estimation of the amount of oxidizable material present within the
sample via oxidation with a strong acid (Eaton et al.,
2005
), and from this the amount of
organic matter removal is estimated. The focus of this study was the removal efficiency
of organic matter as opposed to understanding and interpreting the nature of the observed
fluorescence spectra.
It is now accepted that all wastewaters exhibit characteristic fluorescing properties and
this phenomenon was first reported in the mid-1990s. Research undertaken by Ahmad et al.
(1994), Ahmad and Reynolds (
1995
), and Reynolds and Ahmad (1995) demonstrated the
fluorescence emission spectra of wastewaters using a number of different excitation wave-
lengths. A typical fluorescence emission spectrum, using an excitation at 280 nm, is shown
in
Figure 3.13
. From these early studies, research concerning the use of fluorescence as
a tool for water treatment process optimization, water quality assessment, and pollution
monitoring has emerged (Henderson et al.,
2009
). Further developments from this early
work facilitated the use of synchronous fluorescence spectroscopy (SFS), which is the
simultaneous scanning of both the excitation and emission wavelengths, and the recording
of the distribution of intensities over the emission (or excitation) wavelengths.