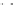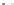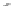Geoscience Reference
In-Depth Information
Let us again start with the continuous plane of atoms added second
and represent it in grey (Fig. 2.3). Then let us introduce above this the
third plane of atoms, drawn as dotted circles. In this plane, starting with
hexagonal close packing, remove one atom out of two, one row out of
two. It is then seen that the superposition of these planes no. 2 and no.
3 define a certain number of tetrahedral cavities pointing downwards. In
each one it is possible to introduce an atom of silicon (drawn in black).
Large cavities bound by six atoms also appear, the
hexagonal cavities
. The
two planes of atoms together define a
tetrahedral sheet
or 'T'.
Hexagonal vacancy (no atom in
the upper level)
Tetrahedral cavity
Oxygens of the upper plane (no. 3)
Silicon
Oxygens of the lower plane (no. 2)
Fig. 2.3
Tetrahedral sheet seen from above and cross-sectional view of an isolated
tetrahedron.
But it is seen that for obtaining this representation, the atoms of
plane no. 2 (in grey) have been widely separated from one another.
If this device is not used, octahedral cavities will be obtained by
superposition of planes 2 and 3, which is not true in clay minerals.
Many authors neglect this. But it is true that for taking this into account
and establishing the mechanical impossibility of this superposition, the
computer must be used. In other words, the representation of atoms that
we give at visible scale is very approximate, the steric packing of anions
not being the same in all planes (R. Calvet, pers. comm.).
This is why it is undoubtedly wiser to limit oneself to representation
in section, still more schematic than the preceding ones (Fig. 2.4).
Clay minerals having one tetrahedral sheet and one octahedral sheet
are designated '1/1', whereas those containing two tetrahedral sheets are