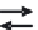Geoscience Reference
In-Depth Information
acid pH, below 5.0, except in thick E horizons of tropical soils
in which quartz is left and where the pH rises to 6.2 (Schwartz
1986, 1988b);
apparently very low base saturation; this is linked to the overes-
timation of the cation exchange capacity (CEC), at least when it
is determined by the standard method at pH 7; we have already
mentioned this (Chap. 10, § 10.4.2);
free iron content (Fe
o
, Table 11.4) with maximum in the Bs and
a free aluminium content with maximum in the same horizon
or just below it, in the upper part of the C horizon. The iron
colours the profile yellow or brown, while aluminium oxides are
colourless and so cannot be detected morphologically.
Metal-organic complexes
are associations binding the metals (iron and
aluminium) to organic acids. The acids liable to capture these metals
are called
complexing agents
.
Some authors distinguish metal-organic
complexes
(binding of the
metal ion to just one acid functional group) and metal-organic
chelates
,
in which several reactive sites are involved. But in current usage, the
term
complex
includes both. Figure 11.3 presents examples.
Concepts of chelates and complexes
O
O
Chelate of
aluminium
C
O
-
C
O
+
A1
3+
+
2 H
+
+
H
2
O
Al
OH
OH
O
O
O
Complex of iron
+
H
+
O
-
+
Fe
2+
+
H
2
O
R
C
R
C
O
Fe
OH
Fig. 11.3
Examples of chelate of aluminium and complex of iron (Schnitzer 1984).
The groups having complexing action are, in order of decreasing
affinity for the metal ions:
-O-, -NH
2
, -N=N-, =N (aromatic), COO-, -O-, C=O
In some cases, complexation involves a hydrogen bond.
Apart from aluminium and iron, many other metals are liable to be
complexed (Pb, Cr, etc.). But the special attention given to Al and Fe is