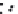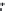Geoscience Reference
In-Depth Information
the content of Ca
++
ions increases (if the carbonate-rich solution
reaches the non-decalcified B horizon),
the partial pressure of CO
2
diminishes (e.g., reduction of biological
activity),
the concentration of Ca
++
ions rises through disappearance
of water (e.g., movement of the carbonate-bearing solution
towards a drier environment through evaporation, root suction,
cryodesiccation, lowering of the water table, etc.).
These phenomena are not independent of each other. For example,
loss of water improves the circulation of air in the pores, favouring
elimination of CO
2
. When crystallization is rapid, the crystals of calcite
are small and numerous (
micrites
). When it is slow, the crystals have
time to grow larger (
sparites
).
7.4.2 Calcretes, Caliches
Calcium-carbonate crusts, called
calcretes
or
caliches
and also
petrocalcic
horizon are observed in regions having a humid season (mobilization of
calcium) and a dry season (deposition). This obtains in the Mediterranean
environment: southern France and more so the Maghreb (Laouina and
Vaudour 1998), also California, South Africa, southern Australia… Their
thickness is often a few cm, but could reach 3 m. Long ignored, their study
became pertinent because they can give information on palaeoclimates:
18
O/
16
O provides palaeotemperatures and the uranium/thorium method,
the corresponding dates.
Generalities
A simplified scheme of calcrete formation is given in Figure 7.14.
Lateral water
circulation
++
Ca
Petrocalcic
horizon
Laminar
crust
Diffuse accumulations
(mottles)
Discontinuous
accumulations
Dominantly massive
accumulation
Fig. 7.14
Simplifi ed scheme of formation of a calcareous crust in a calcareous soil.