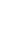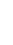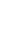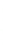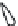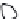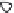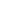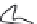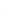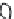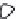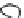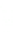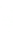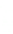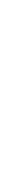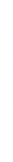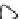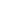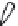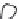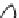Geoscience Reference
In-Depth Information
7.2.4 Role of Topography
The role of geomorphology is very important. The factors favourable for
strong acidification are:
High stability of the rock which impedes the rubbing of calcareous
elements against each other. For example, the magnificent screes
of the col de l'Izoard (southern Alps) are decarbonated only
in the areas where they are perfectly stabilized by the plants
(Fig. 7.6, case 2 versus case 3).
Sufficient distance from the calcareous topslope. A scree
dominated by a limestone cliff has its surface nourished by
limestone debris and does not get acidified.
A location that is not fed by soil solutions saturated with
calcium.
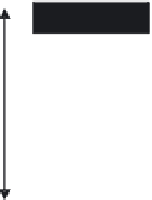
3: in an
unstable
scree:
soil
rich in
calcium
carbonate in
its mass (base
saturation =
100%)
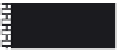
1: on hard calcareous
rock:
humic and dystric
soil near the surface
(base saturation <50%)
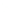
2: in a stable scree:
Humic soil with base
saturation >50%
Fig. 7.6
Types of soil as a function of the distribution of carbonate fragments. Calcareous
materials (in white) and pedological residue (black or dotted). The persistence of calcium
carbonate in the fi ne earth favours mineralization of organic matter (case 3).
7.2.5 Biotic Factors
In France, it is common to see calcareous soils in cultivated regions and,
contrarily, decarbonated soils in the neighbouring forests. The plough
has risen and pulverized the pebbles! Wherever man is absent, the soil
will be decarbonated or acidified in humid climate.
Under natural vegetation, it is observed that acidification is slowed
down in the thin soils colonized by roots. The roots extract calcium
on contact with the rock and ensure its biological recycling via the
stalks or trunks, leaves or needles and then annual residues. But this
limited recycling concerns mostly the surface layers (Michalet and