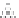Geoscience Reference
In-Depth Information
DESILICATION IN THE NATURAL ENVIRONMENT
Silicates, for example orthoclase
with Si/Al = 3 atomic ratio
(
)
Bisiallitization
Illite, montmorillonite and chlorite
with 3 > Si/Al > 1
Mineral species
dominant in
temperate zone
Monosiallitization
Kaolinite:
Si/Al = 1
a silica tetrahedron
an octahedral sheet
Allitization
Gibbsite:
Si/Al = 0
true hydrolysis
limited
transformation
Species
dominant in
Ferralsols
Fig. 3.9
Weathering of silicates in humid, welldrained and non-acid environment (complete
or partial hydrolysis and controlled transformation).
Table 3.4
Mean composition of waters draining from Ferrallitic soils (Lucas
et al
. 1996, after Cornu 1995).
Si (mg L
-1
)
Al (mg L
-1
)
pH
Lysimeter waters
0.73-1.5
0.11-0.54
3.9-4.8
Ground waters
1.1-1.8
0.05-0.13
4.6-5.0
Quartz is attacked because its solubility is 6 mg l
-1
at 25° C
and the waters are undersaturated with respect to it. But these are
environments in which the mixture quartz + gibbsite + kaolinite is
dominant. Thermodynamic modelling shows that the waters, in tropical
environments, are theoretically supersaturated with respect to gibbsite
and kaolinite in the 'log Al
3+
vs log H
4
SiO
4
' diagram shown later (Bourrié
et al
. 1989). It is therefore normal that these two minerals appear as the
final and practically stable products of weathering. But in reality a
little aluminium is exported in the water, as we saw above. In addition,
kaolinite is destroyed when it is introduced as test mineral in these soils.
In short, these two minerals can also disappear from them.
In temperate environment, weathering is often halted at the stage
of 2/1 clay minerals, the bisiallites that have one octahedral sheet