Geoscience Reference
In-Depth Information
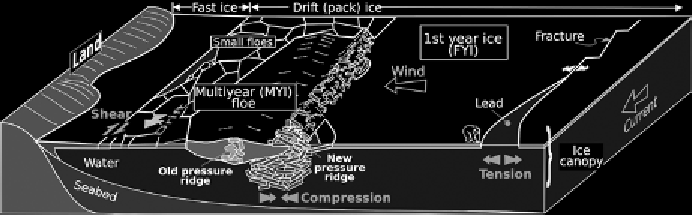
Figure 7.1.
Schematic of basic sea ice types, morphology, and processes (Wikimedia
Commons, author Lusilier, licensed under the Creative Commons Attribution-Share
Alike 3.0 unreported license). (See plate section for color version.)
river input into the Arctic Ocean. There is evidence that a change in the
freshwater output via the Fram Strait could reduce salinities in the North
Atlantic and alter deepwater production; this seems to have occurred in
the late 1960s and 1970s during the so-called Great Salinity Anomaly.
The sensitivity of deepwater production to such processes, however,
remains in debate. As reviewed in
Chapter 10
, the last glacial cycle was
characterized by periods when North Atlantic deepwater production either
ceased or was greatly reduced, albeit from very different processes.
7.1
Sea Ice Formation, Growth, and Melt
7.1.1
The Existence of the Sea Ice Cover
To build on concepts introduced in
Chapter 2
, it is useful to draw from the review
of Maykut (
1985
) and compare the processes of ice formation in a freshwater body
with those that occur in the Arctic Ocean. A water molecule has one oxygen and
two hydrogen atoms, strongly joined by covalent bonds. Water molecules are in
turn attracted to each other by weaker hydrogen bonds that exist between the posi-
tively charged hydrogen atoms and the negatively charged oxygen atoms of neigh-
boring water molecules.
Figure 7.2
gives the temperature versus density relation-
ship for fresh water. For most substances, decreased temperature results in higher
density. But fresh water is an unusual substance. Down to certain temperature,
cooling results in increased density. Below this temperature of density maximum
(T
m
) of 3.98°C, further cooling results in lower density. The solid form of water
is in turn roughly 10 percent less dense than liquid water at the freezing point,
which is another way of saying that ice floats. The reason this occurs is that as the
water cools below the temperature of maximum density, the disorganized hydro-
gen bonds adjust to hold the negatively charged oxygen atoms apart, producing an
organized crystal lattice (ice), which - for a given mass - occupies a larger volume
than liquid water.