Geoscience Reference
In-Depth Information
limited by grain-boundary diffusion in solid-state
is the predominant deformation mechanism of
the ''dry'' silicate mantle. However, deformation
of rock wetted by solvent occurs by a pressure-
solution creep, which is limited by silicate dif-
fusion in the liquid (Weyl, 1959). The principle
of this mechanism is as follows: stress concentra-
tion at the grain contacts causes local dissolution,
diffusion of the dissolved material out of the in-
terface, and deposition at the less stressed faces of
the grains. Since the diffusion in the liquid is sev-
eral orders of magnitude faster than that in solid,
the pressure-solution creep will be much faster
than diffusion creep. The viscosity of wetted rock
is given by:
than 250 km, and is reduced to immobile diamond
or carbide since the mantle redox conditions cor-
respond to the stability of (Fe,Ni) metal. In our
model, most of carbonatite melt at the slab-
mantle interface would segregate into magma
diapir rather than disperse into surrounding man-
tle (Figure 2.15b). Moreover, since the carbonate
reservoir is replenished by continuous subduc-
tion, the limited amount of iron in the mantle
above the slab may be totally oxidized with time.
Most extensive redox interaction is expected at
the beginning of carbonate melting, when the
first diapir rises up through the pristine Fe
0
-
bearing mantle from about 550 to 250 km depth.
All metallic iron on the route of this diapir
would inevitably react with carbonatite melt:
2Fe
d
3
RT
ADC
0
Mw
,
η
Wet
_
mantle
=
(2.7)
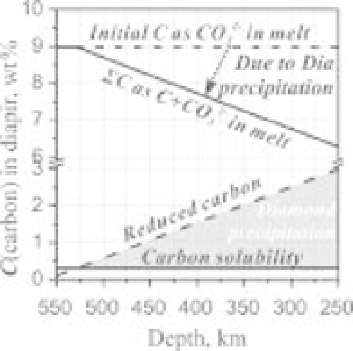
C. Carbon solubility in
carbonate melt is about 0.3wt% at the man-
tle conditions (Palyanov
et al
., 2005). Therefore,
carbonatite melt soon becomes supersaturated
with carbon, which causes diamond precipita-
tion (Figure 2.16). Reduction of CO
2
should also
be accompanied by silicate precipitation from the
melt. This ''redox freezing'' gradually reduces size
of the carbonatite diapir and its ascent rate by
about 30% and 50%, respectively, for an initial
+
CO
2
→
2FeO
+
where
A
is the constant of about 10 for equiax-
ial polycrystals (Vickers & Greenfie, 1967;
Kruzhanov & Stockhert, 1998),
C
0
is the silicate
solubility in carbonatite melt (silicate mole
fraction is about 0.24 at temperature of adiabat),
M
is the molar volume of silicate (3.93 m
3
/mol
for wadsleyite (Katsura
et al
., 2009)). Following
the fluid film model, the effective grain boundary
width,
w
, is of the order of 1-10 nm and the
diffusion coefficient of solute in film is the
same as an order of magnitude lower than that
in the bulk melt (Dysthe
et al
., 2003). Such
an analysis yields a viscosity of 8
10
16
Pa s
providing the ascent rate of 0.5m/year for
r
×
=
1 km (Figure 2.15a). Diapirs with such ascent
rate could consume all carbonatite, which could
be potentially extracted from the subducting
slab (Figure 2.15b). Nominally, rising diapirs
could support an upward CO
2
flux, two orders of
magnitude higher than the subduction CO
2
flux.
Note that the maximum rate of diapir ascent
through the ''dry'' mantle cannot exceed the
infiltration rate. According to our preliminary
estimations this rate approaches to 0.5m/year
(Figure 2.15a). However, diapirs following behind
the first one would not have such a rate limitation
if carbonatite saturated conduits are established.
Rohrbach & Schmidt (2011) suggested that car-
bonatite melt is unstable, when at depths greater
Fig. 2.16
Carbon loss of the carbonatite melt during
diapiric ascent due to carbonate reduction by metallic
iron dispersed in the mantle. We assume
C
(Fe
0
)
0.1 wt % in the depth range of 660-250 km
(Frost
et al
., 2008).
=