Geoscience Reference
In-Depth Information
are similar in composition to those in the systems
withH
2
O due to the enhanced thermal stability of
carbonates relative to hydrous silicates. Basaltic
melts are also formed in systems with reduced
fluid (Jakobsson & Holloway, 2008; Litasov
et al
.,
2013b).
The brief review of melt compositions above
indicates that in most environments low degree
partial melting of volatile-bearing mantle litholo-
gies leads to formation of silicate melt, with
compositions close to basaltic magma. Forma-
tion of carbonatite- or kimberlite-like melts is
possible only under relatively H
2
O-poor oxidized
conditions (in the stability field of carbonates or
H
2
O-CO
2
fluid with molar H
2
O/(H
2
O
C
O
2
)ra-
tio less than 0.3). This suggestion is consistent
with low concentrations of H
2
O(
+
20-50 ppm) in
minerals from diamondiferous xenoliths and in-
clusions in diamonds (Matsyuk & Langer, 2004;
Matveev & Stachel, 2007).
<
2.8 Redox Melting, Redox Freezing, and
Diamond Formation
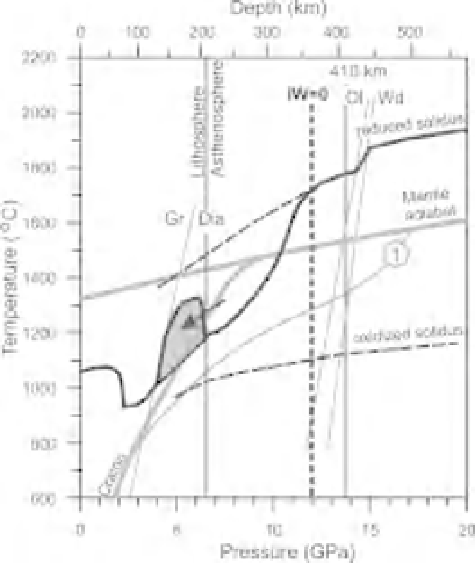
Fig. 2.12
Mantle solidus at the contact of oxidized
asthenosphere and reduced cratonic lithosphere. The
arrow show metasomatic or magmatic modification of
cratonic roots (gray field) and crystallization of
asthenosphere melt by interaction with reduced and
depleted cratonic peridotite. This would be the major
mechanism of diamond crystallization in cratonic
roots. The PT-profiles and solidi are from Figures 2.3,
2.6, and 2.10.
Although some diamonds contain native iron
(Sobolev
et al
., 1981), they cannot be in
equilibrium with Fe at mantle PT-conditions.
Fe-carbides (Fe
3
CandFe
7
C
3
) are stable at any
PT-parameters below the solidus of Fe-C system
(e.g. Scott
et al
., 2001). The assemblage of Fe and
diamond is possible only if Fe is in the molten
state and carbon solubility in this metal melt is
limited. Accordingly, the presence of diamond in
the mantle including parts of the lower mantle
levels suggests the absence of native iron there.
This supports the idea that at least some regions
in the mantle are more oxidized than expected
from standard models described above. The slow
rate of exchange processes in the mantle may be
consistent with super-deep diamond formation
in oxidized ancient subduction domains.
Our data for melting in volatile-bearing sys-
tems constrain the conditions for redoxmelting in
the Earth's mantle. Classic redox melting implies
oxidation of CH
4
-H
2
O fluid, which causes forma-
tion of H
2
OandCO
2
and drastically decreases
the melting temperature of rocks (Figure 2.12).
Recently, Foley (2011) suggested that two types
of redox melting operate in the mantle. He called
the classic mechanism hydrous redox melting
(HRM) and emphasized the importance of carbon-
ate redox melting (CRM). The HRM corresponds
to the transition from methane to hydrous flu-
ids (at
fO
2
=
=
+
1.5) and character-
izes ancient subduction, lithosphere erosion and
metasomatism. The CRM corresponds to transi-
tion from hydrous to CO
2
-fluids (at
fO
2
=
IW
0-IW
IW
+
4.5 - IW
5.5 or FMQ-1.5 - FMQ - 0.5) and is
typical for Phanerozoic subduction and magma-
tism. An important observation is the extension
of the region of the so-called water maximum
with increasing pressure. At high pressure (above
+