Geoscience Reference
In-Depth Information
Peridotite
Eclogite
(a)
(b)
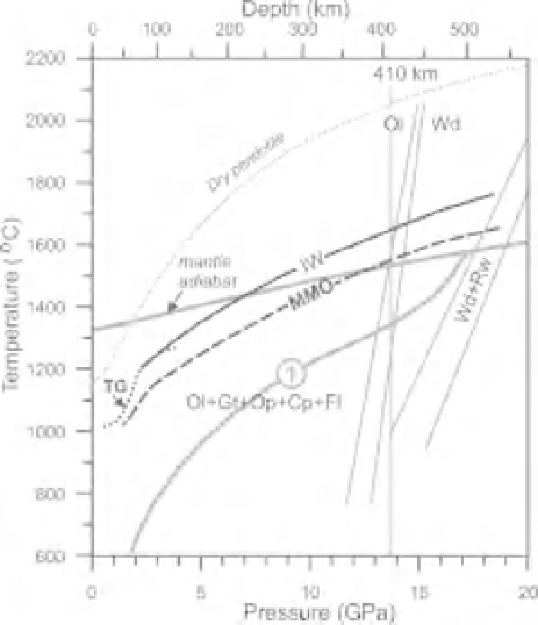
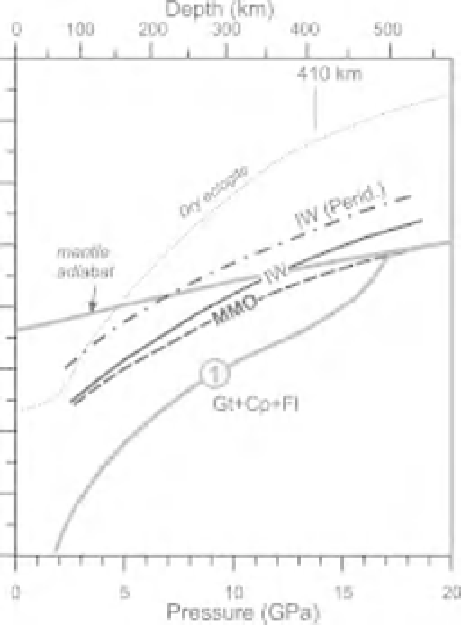
Fig. 2.10
Solidi in the systems peridotite-C-O-H fluid (A) and eclogite-C-O-H fluid (B) at
fO
2
buffered by MMO
(Mo-MoO
2
) and IW (Fe-FeO). TG, Solidus in the system peridotite
1 (Taylor &
Green, 1988) (Figure 2.5). For comparison a dash-and-dot line in B indicates the peridotite solidus buffered at IW
from A. Gray lines indicate the main phase transitions, volatile-free solidi, and mantle geotherms from Figure 2.3.
Ol, Olivine; Gt, garnet; Op, orthopyroxene; Cp, clinopyroxene; Wd, wadsleyite; Rw, ringwoodite; Fl, fluid.
Reproduced with permission of Nature.
+
C-O-H fluid at fO2
=
IW
+
compositions (Ghosh
et al
., 2009; Kiseeva
et al
.,
2013). The factors determining the position of the
solidi at pressures above 6GPa in systems with
H
2
OandCO
2
reveal fundamental differences. In
the H
2
O-bearing systems, the solidus depends on
the H
2
O solubility in nominally anhydrous sil-
icates. The solidi in the system peridotite-H
2
O
within the transition zone can lie 300-400
◦
C
above those of H
2
O-bearing eclogite because of
the high H
2
O solubility in wadsleyite and ring-
woodite (Figure 2.3). The position of the solidus in
CO
2
-containing systems depends on the presence
of alkalis and H
2
O. A small amount of K
2
O can re-
duce the solidus temperature of carbonate-bearing
eclogite or peridotite by 400-500
◦
Cat20GPa.
The CO
2
content of the system itself should not
have significant influence on the solidus. The sta-
bility of carbonates (magnesite, aragonite) is not
significantly dependent on the alkali content, but
decreases dramatically if H
2
O is added to the sys-
tem (Figure 2.7). In peridotite and eclogite systems
with a hypothetical C-O-H fluid, the solidi are
at higher temperature than for systems with CO
2
and H
2
O. Nevertheless, they are still substan-
tially (300-400
◦
C) lower than the ''dry'' solidi at
16GPa (Figure 2.10). Under reduced conditions,
prevalent in the most mantle, the first melt for
PT-profiles between the average adiabat and sub-
duction would be a metallic liquid in the Fe-S-C
system (Morard
et al
., 2007; Nakajima
et al
.,