Geoscience Reference
In-Depth Information
1
1450
H
2
O
0.9
1425
0.8
1400
0.7
1375
CH
4
0.6
1350
0.5
1325
0.4
CO
2
1300
0.3
1275
0.2
H
2
1250
0.1
CO
0
1225
3
45678
9 0 1 2 3
14
1200
Pressure (GPa)
Pressure (GPa)
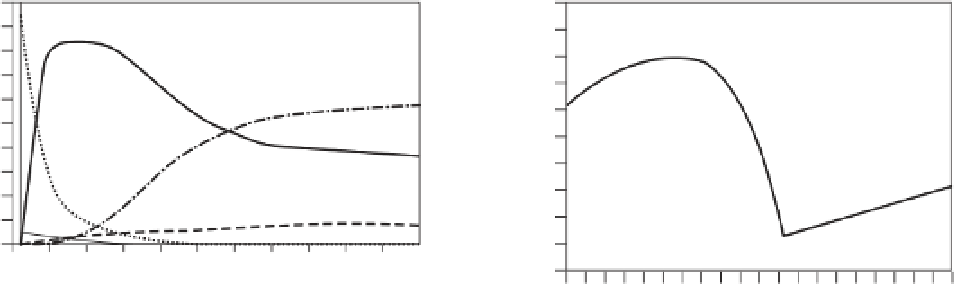
Fig. 1.11
Calculated speciation in a C-H-O fluid as a
function of pressure along an adiabat with a potential
temperature of 1200
◦
C. Oxygen fugacity decreases
with pressure. At about 3 GPa, graphite precipitates
and at higher pressures, fluid speciation is controlled
by the CCO buffer. After Frost and McCammon (2008).
Fig. 1.12
CO
2
-saturated peridotite solidus in the
system CaO-MgO-Al
2
O
3
-SiO
2
-CO
2
(CMAS-CO
2
) Note the sharp drop in solidus
temperature around 2.5 GPa. At lower pressures, a
CO
2
-rich fluid phase coexists with silicate minerals
below the solidus and the first melts are silicate-rich.
At higher pressures, carbonate is a stable phase below
the solidus and the first melts are carbonatitic. After
Dalton and Presnall (1998) and references therein.
Diagram courtesy of Shantanu Keshav.
decreases with depth. The most obvious observa-
tion is that CH
4
increases at the expense of CO
2
with increasing depth. In the presence of sulfide,
H
2
S could also become a significant fluid species.
CH
4
-rich fluids will probably dissolve less sili-
cates than H
2
O-rich fluids or H
2
O-CO
2
mixtures.
Moreover, dilution of H
2
ObyCH
4
could reduce
water solubility in nominally anhydrous miner-
als to such an extent that dehydration occurs.
However, the calculations shown in Figure 1.11
rely on far extrapolations of thermodynamic data,
particularly for CH
4
, so that the details of fluid
composition and fluid properties are uncertain,
although the general trends shown are probably
reliable.
2010). The traces of carbonatite melt are probably
important agents of mantle metasomatism (Green
& Wallace, 1988; Rudnick
et al
., 1993). Gaillard
et al
. (2008) suggested that they may also be
responsible for the high electrical conductivity
observed in some areas of the mantle. However,
the high carbon contents in carbonatite melt to-
gether with the low bulk mantle abundance of
carbon probably limit the effect these traces of
melt may have on observed conductivities.
In the seismic low velocity zone, carbon may
enhance the melting point depression caused by
water and may dissolve in the hydrous melts
(Hirschmann 2010). At greater depths in the man-
tle, particularly in the transition zone and lower
mantle, carbonates are not stable any more and
carbon occurs in reduced form (as diamond, car-
bides or dissolved in iron metal) and therefore
has little effect on solidus temperatures. Upon
upwelling from the deep mantle, reduced car-
bon will be converted to carbonates or CO
2
and
therefore produce small degrees of carbonatite or
1.4.5 Carbon, melting in the mantle and the
deep carbon cycle
The CO
2
saturated peridotite solidus (Figure 1.12)
drops sharply around 2 GPa, where carbonates be-
come stable (Wyllie & Huang, 1976; Eggler, 1978;
Dalton & Presnall, 1998; Gudfinnsson & Presnall,
1996). Since carbon is so extremely incompatible
in mantle minerals, small degrees of carbon-
atite melt will be present throughout wide parts
of the upper mantle (Dasgupta & Hirschmann,