Geoscience Reference
In-Depth Information
Volcanic Arc
Backarc
Trench
0
0
amp
+
Chl
+
Serp
pyroxenites
1
spinel peridotite
amp
+
H
2
O
Cc
Dol
Mag
Amp
Talc
amphibole-peridotite
50
1000
C
2
°
garnet peridotite
1300
C
amp-free
peridotite
+
silicate melt
°
3
peridotite
+
cb-melt
100
Zo
flow lines
4
Cld
phlogopite-peridotite
5
1
50
Chlorite
6
Phlogopite
K-Richterite
Antigorite
200
7
10A
8
“A”
250
devolatilization
9
section of slab
dehydration
section of slab melting
Lawsonite
10
300
Phengite
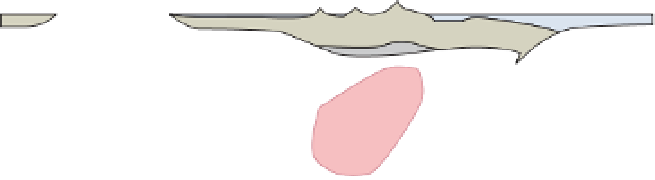
Fig. 1.9
Dehydration reactions and melting in subduction zones. Diagram from Bali (2004), after Schmidt and Poli
(1998). Reproduced with permission of Elsevier.
While the processes occurring in subduction
zones are understood in principle, there are major
uncertainties in the effect of water on man-
tle solidus temperatures and on the composi-
tions of primary melts in mantle peridotites.
These uncertainties are largely related to exper-
imental difficulties, in particular to quenching
problems. While in felsic (e.g. granitic) systems
below 1 GPa, hydrous melts can be quenched
to glasses that are easily recognized and an-
alyzed, this is not possible in peridotitic sys-
tems under upper mantle pressures. Here, the
hydrous melts crystallize upon quenching. Dis-
tinguishing quench crystals from residual crystals
that were never molten is difficult and obtain-
ing accurate compositions of melts is even more
difficult. Moreover, hydrous fluids dissolve a sig-
nificant amount of silicates at high pressures
and temperatures, which will precipitate as crys-
talline material during quenching. Distinguish-
ing quenched solute from hydrous fluids from
quenched hydrous silicate melts is therefore an-
other problem in these studies. For these reasons,
even the temperatures reported for the water-
saturated solidus in peridotite at shallow upper
mantle pressures differ by several 100
◦
C(Mysen
& Boetcher, 1975; Kawamoto & Holloway, 1997;
Kawamoto, 2004; Grove
et al
., 2006). A plausible
location of the solidus may be close to 1000
◦
C
in the range from 1 to 4-6 GPa, with a critical
endpoint between 4 and 6 GPa, where water and
silicate melt become completely miscible. There
has also been a considerable debate on the ef-
fect of water on primary melt compositions and
whether the primary melts are basaltic or an-
desitic (e.g. Kushiro, 1969, 1972; Green, 1973;
Mysen & Boettcher, 1975; Hirose & Kawamoto,
1995; Liu
et al
., 2006). At least in some simple sys-
tems at pressures around 1 GPa, the effect of water
appears to be to produce quartz-normative melts
instead of olivine-normative melts (e.g. Kushiro,
1969; Liu
et al
., 2006).
Melting at mid-ocean ridges is due to decom-
pression; ascending mantle crosses the dry peri-
dotite solidus and considerable fractions of melt
are being produced. As such, this process does not
require the presence of water. However, water