Geoscience Reference
In-Depth Information
Mosenfelder
et al
., 2005; Withers
et al
., 2011).
While earlier studies (e.g. Kohlstedt
et al
., 1996)
calculated water contents from infrared spectra
using the Paterson (1982) calibration, later work
suggested that this calibration underestimates the
water contents of olivine by about a factor of
three (Bell
et al
., 2003). If this factor is taken into
account, there is very good mutual consistency
between the available studies. Water solubility in-
creases continuously with pressure; Mosenfelder
et al
. (2005) observed 6399 ppm H
2
Obyweight
at 12 GPa and 1100
◦
C. In addition, water solubil-
ity also increases with temperature (Zhao
et al
.,
2004; Smyth
et al
., 2006), but at very high temper-
atures, observed water contents start to decrease
again. This effect is likely related to a decrease of
water activity in the coexisting fluid phase, not
to an intrinsic reduction of water solubility in
olivine. Smyth
et al
. (2006) found a maximum of
8900 ppm water in olivine at 12 GPa and 1250
◦
C.
Water solubility in orthopyroxene is very dif-
ferent from olivine. This is because in orthopy-
roxene, water solubility mostly involves coupled
substitutions with Al
3
+
,suchasAl
3
+
and H
+
replacing Si
4
+
or Al
3
+
+
Water solubility in pure MgSiO
3
enstatite is
relatively low (Mierdel & Keppler, 2004), but
increases greatly with Al (Rauch & Keppler, 2002;
Mierdel
et al
., 2007). If orthopyroxene coexists
with olivine and an Al-rich phase such as spinel
or garnet, the Al-content of the orthopyroxene
is buffered. The water solubility in such an Al-
saturated orthopyroxene decreases strongly with
both pressure and temperature. This is probably
partially due to the fact that the substitution of
the larger Al
3
+
cation on the Si
4
+
site becomes
unfavorable at high pressure. The contrasting
behavior of water solubility in olivine and in
orthopyroxene produces a pronounced minimum
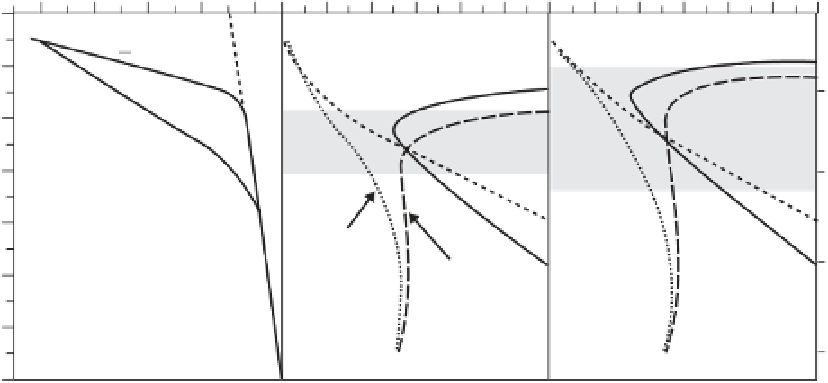
in water solubility in the upper mantle, which co-
incides with the depth of the seismic low velocity
zone (Figure 1.3). The minimum in water solubil-
ity implies that water partitions more strongly
into melts and therefore likely stabilizes a small
fraction of partial melt in the seismic low velocity
layer (Mierdel
et al
., 2007).
At transition zone and lower mantle pressures,
water solubility in minerals is not always easy
to define, because the minerals usually coexist
with a very solute-rich fluid or with a hydrous
replacing 2 Mg
2
+
.
H
+
Water solubility (ppm H
2
O)
Water solubility (ppm H
2
O)
Temperature (
°
C)
600
1000
1400 0
500
1000 1500 2000 2500
0
500
1000 1500 2000 2500 3000
0
continental
oceanic
60% olivine
+
40% AI-saturated enstatite
20
100
40
LVZ
olivine
60
LVZ
200
80
pure
enstatile
300
AI-saturated
enstatite
100
upper mantle adiabat
120
400
140
Fig. 1.3
Water solubility in the upper mantle as a function of depth, for an oceanic and a continental geotherm.
LVZ
=
seismic low-velocity zone. Modified after Mierdel
et al
. (2007). Reprinted with permission from AAAS.