Geoscience Reference
In-Depth Information
than helium in the Solar Nebula, magnesium
(Mg), iron (Fe), silicium (Si), aluminum (Al),
titanium (Ti), and calcium (Ca) bound to oxygen
(O), are the constituents that make up most of the
terrestrial planets in the Solar System. Given that
these elements are also abundant in other stars
and thus in the solar nebulas from which they
accreted along with their planets, it is reasonable
to expect rocky super-Earths to be composed of
the same elements. One caveat is that planets
that form around carbon rich stars (C
/
O
>
0.8,
Bond
et al
., 2010) may be composed of carbide
minerals instead of oxides. However, the planets
found around these stars are so far gas giants, and
it is difficult to infer the composition of their
solid interiors as they contribute very little to the
total mass.
Provided that the data available is only
M and R, super-Earths' structure can be largely
reproduced by considering only Mg, Fe, Si, and
O. Internal structure models consider a silicate
mantle above an iron rich core. In some models
the mantle is layered to: olivine and pyroxene
(Mg, Fe)
2
SiO
4
+
40000
HP-26b
500 K < T
eq
< 700 K
700 K < T
eq
< 900 K
900 K < T
eq
< 1100 K
1100 K < T
eq
< 1300 K
1300 K < T
eq
< 1500 K
1500 K < T
eq
< 1700 K
1700 K < T
eq
< 2200 K
K18-c
K11e
HP-11b
30000
U
N
K4-b
K11d
GJ 436b
K11c
20000
K20c
100
env
GJ 1214b
K11f
55 Cnc-e
K18-b
50%
K11-b
K20-b
10000
C7-b
K10-b
8000
5%
6000
T
eq
= 600 K
T
eq
= 500 K
5000
0.4
0.7
1
2
3
5
7 10
20
Mass [M
Earth
]
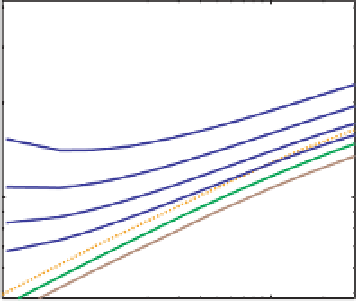
Fig. 9.6
Mass-Radius relationships for warm vapor
planets. Data for planets are shown color-coded by
their equilibrium temperature. The mass-radius
relationships (dark blue) correspond to compositions of
different amounts - 5, 20, 50, 100% by mass - of a
pure-water envelope above an Earth-like nucleus for
equilibrium temperatures of
500 K and 600 K,
relevant to GJ 1214b, Kepler-11f and Kepler-11e. The
100% pure-water composition is the boundary above
which planets of the corresponding equilibrium
temperature or above require H-He. (See Color Plate 8).
∼
(Mg, Fe)
2
SiO
6
present in the
upper mantle, higher pressure forms of olivine
in the transition zone plus pyroxenes, perovskite
and
(Mg, Fe)O
in the lower mantle, post-perovskite and mag-
nesiowustite
magnesiowustite
(Mg, Fe)SiO
3
+
Determining the planetary composition is a
degenerate problem, more acute in the case of
the solid planets - many different compositions
can fit the data. While the gas giants are mostly
composed of (H) and helium (He) atmospheres
that dominate the structure and the radius of
the planet, the building blocks of super-Earths
are more numerous and complex. They are rocks
(iron cores, silicate oxide mantles), H
2
Ointhe
form of ices, water or vapor, and in the case of
mini-Neptunes also some H-He. There could be
other constituents such as other ices (ammonia,
methane, etc.), but the most abundant are the
ones mentioned above. Therefore the problem
has two known parameters (mass and radius),
and several unknowns (the building blocks). This
means that it is not possible to determine a
unique planetary composition but instead one
defines upper and lower limits for the different
constituents, or likely compositions.
(Mg, Fe)O in the
lower-most mantle. Most internal structure
models for exo-Earths ignore the light alloy in
the core, as it has little impact on the total
planetary radius given the other uncertainties in
the composition. The ''geophysical'' structure
models (Valencia
et al
., 2006, 2007; Sotin
et al
.,
2007; Grasset
et al
., 2009) incorporate a more
realistic mineralogy for the mantle, while the
''astrophysical'' structure models (Seager
et al
.,
2007; Rogers
et al
., 2010a; Fortney
et al
., 2007)
use a simplified composition of just perovskite,
or olivine for the mantles. See Figure 9.4 for
an example of the density profile of Earth-like
planets.
In 2004 Murakami
et al
. (2004) discovered a
new high-pressure phase of magnesium silicate
oxide, post-perovskite, relevant to the deepest
portion
(Mg, Fe)SiO
3
+
of
the
Earth's
mantle.
This
phase
(a) Composition
Because of their high abun-
dance with respect to the other elements heavier
becomes stable at pressures higher than
126
GPa, so that in contrast to the Earth that has
∼