Geoscience Reference
In-Depth Information
8
Ideal mixing
Fe-Si melt at 4 GPa
Tateyama
et al
. (2011)
0
Fe-Si melt at 1 atm
Dumay & Crame (1995)
Fe-Si melt
−
1
Fe-Si melt at 4 GPa
Tateyama
et al
. (2011)
6
−
2
Fe-S melt
−
3
4
Fe-S melt at 4 GPa
Nishida
et al
. (2008)
−
4
Fe-S melt at 4 GPa
Nishida
et al
. (2008)
−
5
2
0 0 0 0 0 0
X at%
0 0 0 0 0 0
X, at%
(a)
(b)
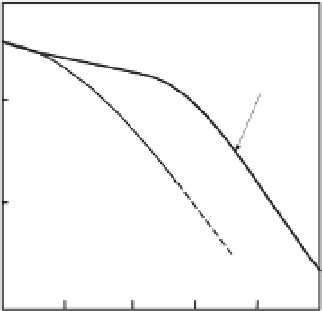
Fig. 8.17
(a) The compositional dependency of the density of Fe-S and Fe-Si liquids at high pressure at 4 GPa and
1923 K (Nishida
et al
., 2008; Tateyama
et al
., 2011), and (b) the compositional dependency of the excess volumes of
mixing of the liquids at the same condition. The excess volume of the Fe-Si melt at one atmosphere (Dumay &
Cramb, 1995) is also shown in this figure. Reproduced with permission of Springer.
2008; Tateyama
et al
., 2011). The excess volume
is deviation of the volume of the liquid from the
linear mixing of the volume of the end member
components, which indicates the ideal behavior
of the melt. Thus, the excess volume is a mea-
sure of nonideality of the liquid. These figures
clearly illustrate the nonideal behavior of these
liquids at around 4 GPa, which is consistent with
the behavior of these liquids at atmospheric pres-
sure. A negative volume of mixing indicates that
the density decrease is smaller in these liquids
compared with those following an ideal mixing
model, which is usually assumed for estimation
of the light element content from the density
deficit of the outer core. If the nonideality of the
Fe-S and Fe-Si liquids persisted at the Earth's
core conditions, the amounts of light elements in
the liquid outer core would be larger than those
estimated previously by ideal mixtures of Fe and
light elements.
experimental pressure directly from Hugoniot
data using the laws of conservation of mass,
energy, and momentum. Pt is a suitable material
for the pressure scale because: (1) it has a cubic
structure with a simple XRD pattern over a wide
range of pressure and temperature, (2) it has a
high density and thus shows strong XRD lines;
(3) it is more compressible above 100 GPa than
the other transition metals, and therefore we
can make a precise density determination by
XRD, and (4) it can be used as a laser absorber
for laser-heating experiments. Pt pressure scales
based on shock experiments have been reported
by Jamieson
et al
. (1982) and Holmes
et al
. (1989).
Holmes
et al
. (1989) compressed Pt to 660 GPa
in a shock experiment and determined the
300 K isothermal equation of state of Pt using a
complementary first-principles theoretical work.
The Pt scale of Holmes
et al
.iswidelyusedas
a primary pressure scale for diamond anvil cell
experiments. Recently, other Pt pressure scales
were presented by several researchers (Dewaele
et al
., 2006; Dorogokupets & Oganov, 2007; Fei
et al
., 2007; Matsui
et al
., 2009).
Several reports indicate that the Pt scale of
Holmes
et al
. yields slightly higher pressures
than other pressure scales (e.g., Dorogokupets &
8.4
Inner Core: Structure, Composition, and
Physical Properties
8.4.1 Pressure scales to the core conditions
Shock
experiments
provide
a
primary
pres-
sure
scale,
because
they
can
determine
the