Geoscience Reference
In-Depth Information
Ni-S binary phase diagram at ambient pressure
contains more intermediate compounds than its
Fe-S counterpart. Thus, Ni may affect the pres-
sure range of the stability field of iron sulfides
such as Fe
3
S. Ni also affects the phase and melt-
ing relations of Fe-Ni and Fe-Ni-Si systems (e.g.,
Kuwayama
et al
., 2008; Sakai
et al
., 2011b). The
details of the effect of Ni on the phase stability
under the core conditions will be discussed in the
following section of the inner core.
The accretion and core formation conditions
such as oxygen fugacity, temperature, and pres-
sure are very important factors controlling the
nature and amount of the light elements in the
core, i.e., the light element abundance in the core
is a key for understanding the early process of
planetary formation.
made to determine the melting relations of the
iron-light-element systems such as the Fe-Si,
Fe-S, and Fe-S-O systems up to the pressure of
the Earth's core.
(a) Melting criteria in high-pressure experiments
There are several criteria for detecting melting
in high-pressure experiments including diamond
anvil cell experiments. The formation of the den-
dritic texture is an important clue for melting
detection. This texture is formed by rapid cool-
ing. This criterion is commonly used for static
experiments using a multianvil press and also a
piston cylinder apparatus (e.g., Ohtani, 1979). The
compositions of liquid and liquid-crystal parti-
tioning are generally based on the composition of
the quenched liquid with a dendritic texture. A
similar dendritic quench texture is also observed
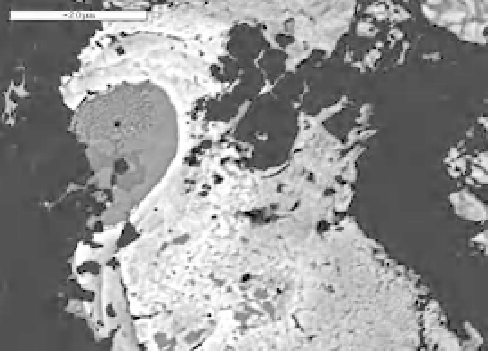
in diamond anvil cell experiments. Figure 8.4
shows the similarity of the dendritic textures
observed in the diamond anvil cell experiments
after laser heating and quenched textures after
high-pressure and high-temperature experiments
made with the multianvil press (e.g., Asanuma
8.3.2 Melting and melt properties of iron-light
element systems
The melting temperature of metallic iron has
been used as a clue to estimate the temperature
profile of the core. The melting temperature of
iron has been determined by various methods
such as static experiments by the diamond anvil
cell (e.g., Williams
et al
., 1987; Boehler, 1993;
Ma
et al
., 2004), shock experiments (e.g., Brown
& McQueen, 1980; Yoo
et al
., 1993; Nguyen
& Holmes, 2004) and theoretical calculations
(e.g., Alfe
et al
., 1999). However, even for pure
iron, there are large differences in melting tem-
perature. Differences exist between the results
determined by the different techniques,but there
are also large differences in the melting tem-
perature determined by different authors using
the same technique. Generally, the theoretical
calculation provides a higher melting tempera-
ture of iron than that determined in the static
experiments.
The core is not composed only of pure iron
but it also contains light elements as discussed
above. The core temperature could be lower than
that estimated previously based on the melting
temperature of pure iron because the light ele-
ments reduce the melting temperature of iron
significantly. Therefore, extensive efforts were
20
μ
m
Solid iron
Dendritic quench
crystal
50
μ
m
Liquid
Solid
Fe-FeS (5 GPa, 1250
°
C) MAP
BSE image
Fig. 8.4
The dendritic textures observed in the Fe-FeS
system quenched from 1500
◦
C at 19.2 GPa after laser
heating in diamond anvil cell experiments (e.g.,
Asanuma
et al
., 2010). The inset is the textures
observed in the sample in the Fe-FeS system quenched
from 5 GPa, 1250
◦
C in the multi-anvil press
experiments (Ohtani, 2009). Similar quench textures
are observed in both experiments.