Geoscience Reference
In-Depth Information
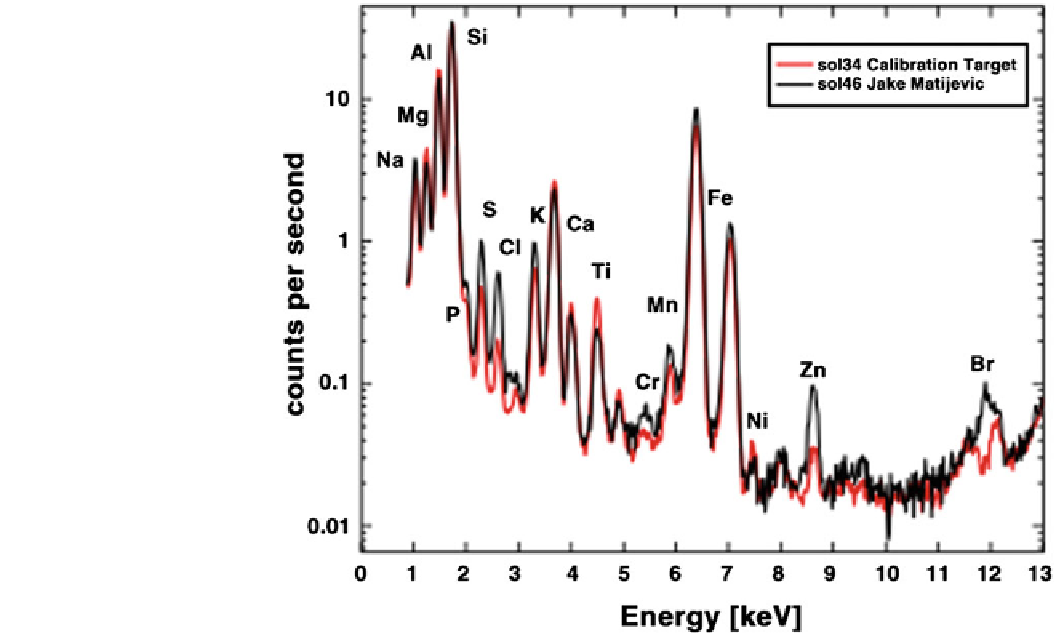
Fig. 17.1
An X-ray spectrum
from the APXS instrument on the
Curiosity rover. A calibration
target is compared with the rock
Jake Matijevic (which may be a
ventifact—see Fig.
16.13
). Com-
pared to previously found rocks
on Mars, the Jake rock is low in
magnesium and iron, and high in
elements like sodium, aluminum,
silicon and potassium which
often found in feldspar minerals.
It has very low nickel and zinc.
The salt-forming elements sulfur,
chlorine and bromine are likely in
soil or dust grains visible on the
surface of the rock. These results
point to an igneous origin. Shar-
per spectra could be obtained
with laboratory analyzers, while
the Pathfinder APXS had a rather
lower resolution. Image: NASA/
JPL-Caltech/University of
Guelph/CSA
parts-per-trillion (ppt, one part in 10
12
). For example,
Zimbelman and Williams (2002) applied ICP MS to sands
from Mojave and the Colorado River, distinguishing two
source regions.
The generation of a plasma from a mineral sample can
also be accomplished with a powerful pulsed laser, and the
Curiosity rover carries an instrument (Chemcam) which
analyzes the light from the plasma (Laser Induced Break-
down Spectroscopy) produced on a sample up to 7 m away
from the rover. Successive laser pulses can be aimed at the
same spot, producing a profile with depth into the target
(thereby differentiating the underlying sample from the dust
that tends to appear everywhere to some extent). Chemcam
observations (they are discussed in this chapter because of
the AES connection, although obviously this is also a field
measurement and in some respects could be considered a
remote measurement, too) have been used to profile the
chemistry in the 'Rocknest' dune deposit.
The methods described above are all elemental abun-
dance techniques. While useful in assessing the provenance
of sands (e.g., one source may be more calcium-rich than
another), they do not indicate the mineralogical composi-
tion, e.g., whether a sample with calcium, silicon and carbon
in it is calcium carbonate plus silica, or a calcium silicate
with diamonds. Some of these possibilities can be discrim-
inated by microscopic examination, but the determination of
mineral composition uses a laboratory technique called
X-ray diffraction (XRD). An X-ray beam is applied to a
field. This approach has been used on spacecraft too,
although most in situ elemental composition measurements
on Mars have used an alpha-particle source to stimulate the
sample. In addition to the X-rays thereby produced by
particle induced X-ray emission (PIXE), abundances of
some lighter elements can be indicated by the energies of
protons that are also produced, and by the energies of alpha
particles that are scattered by the samples, the combined
technique being named Alpha-Proton-Xray Spectroscopy
(APXS). This was the main science instrument on the
Pathfinder rover Sojourner (where it can be seen in action
on the Mermaid ripple in Fig.
16.21
), and has also been
carried on the two Mars Exploration Rovers and the Curi-
osity rover. An example spectrum is shown in Fig.
17.1
.
Somewhat related is the technique of neutron activation
analysis (NAA) wherein a neutron source is used to form
radioactive elements in a sample, which can be detected by
their characteristic emissions of gamma rays. This tech-
nique
is
particularly
effective
for
detecting
very
small
amounts of rare earth elements.
Elemental composition can also be indicated by vapor-
izing mineral samples with extreme heat, most commonly
with an inductively-coupled plasma. Elements can give off
light with characteristic wavelengths (ICP-AES, atomic
emission spectroscopy), essentially a high-tech version of
the bunsen burner investigation. Even greater sensitivity is
obtained by analyzing the vaporizing sample in a mass
spectrometer (ICP-MS) which allows detection of even
Search WWH ::

Custom Search